Two Tonoplast MATE Proteins Function As Turgor-Regulating Chloride
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Master Gardener PUBLISHED by UNIVERSITY of MISSOURI EXTENSION Extension.Missouri.Edu
Master Gardener PUBLISHED BY UNIVERSITY OF MISSOURI EXTENSION extension.missouri.edu Plants and Their Environment David Trinklein, Division of Plant Sciences lants are living organisms that contain chlorophyll and use it to manufacture Ptheir own food. Their cell walls are more or less rigid and support both the individual cells and the whole structure. Even when plants have reached what we regard as their full, mature size, they continue to expand and develop new leaves, flowers, fruit and shoots. Unlike animals, plants cannot move when the environment changes. They are at the mercy of the climate and the gardener because they are rooted in place. Even though it appears that many plants, especially larger ones, are quite tolerant of change, they sometimes do not show adverse effects until long after the event. For example, tree roots are often damaged or killed by suffocation during building projects or flooding. An established tree may still have strength to leaf out and may appear to thrive for several years. But in its weakened state, the tree is more likely to blow down, become infested or simply decline. To understand why plants respond as they do to natural influences and to cultivation, gardeners must understand something about their structure and how they grow. This publication provides such an introduction. Ways to group plants Uses Gardeners tend to group plants by their horticultural uses: fruits, vegetables, flowers, trees, shrubs, turf and so on. These categories are a convenient way to think and learn about plants. Life cycle Plants can also be categorized by the length of their life cycles. -

SAENGWILAI, P.: Effects of Root Hair Length on Potassium Acquisition
Klinsawang et al.: Effects of root hair length on potassium acquisition in rice - 1609 - EFFECTS OF ROOT HAIR LENGTH ON POTASSIUM ACQUISITION IN RICE (ORYZA SATIVA L.) KLINSAWANG, S.1 – SUMRANWANICH, T.1 – WANNARO, A.1 – SAENGWILAI, P.1,2* 1Department of Biology, Faculty of Science, Mahidol University Rama VI Road, Bangkok 10400, Thailand 2Center of Excellence on Environmental Health and Toxicology (EHT) Bangkok, Thailand *Corresponding author e-mail: [email protected] (phone: +66-9-1725-4817) (Received 12th Oct 2017; accepted 20th Feb 2018) Abstract. Potassium (K) deficiency limits rice production worldwide. It has been shown that variation in root traits, such as root hair length, influences ion uptake in many plant species. In this study, we explored natural variation of root hair length of twelve rice varieties in a roll-up system. We found a large phenotypic variation for root hair traits ranging from 0.14 to 0.21 mm. Niaw San-pah-tawng and most upland varieties had long root hairs while lowland varieties had short root hairs. Six lowland varieties contrasting in root hair length were planted in pots under high and low K concentrations. K stress was found to decrease average biomass by 60.93% and K tissue content by 66%. Root to shoot ratio was not affected by K stress. Correlation analysis indicated that long root hair was associated with reduced percentage of leaf senescence, enhanced plant biomass, and improved tissue K content under low K condition. Our results suggest that long root hair could be a useful trait for plant breeding to improve K acquisition in rice. -

Evaluation of the Osmotic Adjustment Response Within the Genus Beta
July 2008 - Dec. 2008 Osmotic Adjustment Response 119 Evaluation of the Osmotic Adjustment Response within the Genus Beta Manuela Bagatta, Daniela Pacifico, and Giuseppe Mandolino C.R.A. – Centro di Ricerca per le Colture Industriali Via di Corticella 133 – 40128 Bologna (Italy) Corresponding author: Giuseppe Mandolino ([email protected]) ABSTRACT Beta genus includes both industrial and horticultural spe- cies, and wild species and subspecies, which are possible reservoirs of agronomically important characters. Among the traits for which Beta has been recently studied, drought tolerance or avoidance is one of the most important. In this work, relative water content and the osmotic potential in well-watered and stressed conditions of three beet types, one B. vulgaris subspecies and one species other than B. vulgaris, all belonging to the Beta genus, were analysed. In addition, relative water content, succulence index and osmotic potential were measured during a three-week water deprivation period, and the osmotic adjustment was estimated for each Beta accession. The results showed that succulence was higher for B. vulgaris ssp. maritima. It was also shown that all Beta accessions were capable of adjust- ing osmotically, but that the B. vulgaris maritima accession examined had a higher osmotic adjustment value compared to the accessions belonging to cultivated Beta types, and that the accession of the wild species Beta webbiana had a comparatively limited capacity to adjust osmotically. Additional key words: Sugarbeet, sea beet, germplasm, drought, osmotic adjustment rought is one of the greatest limitations for agriculture and crop expansion (Boyer, 1982). Sugarbeet (Beta vulgaris ssp. vulgaris) is aD deep-rooting crop, more adapted to withstand water shortage or nutri- tional deprivation than many other crops (Doorenbos and Kassam, 1979; Biancardi et al., 1998); however, drought stress is becoming a major 120 Journal of Sugar Beet Research Vol. -

Learning List 1. Eukaryotic Cells (Plant and Animal Cells) Have a Cell Membrane, Cytoplasm and Genetic Material Enclosed in a Nucleus
Learning List 1. Eukaryotic cells (plant and animal cells) have a cell membrane, cytoplasm and genetic material enclosed in a nucleus. 2. Prokaryotic cells contain cytoplasm, cell membrane and a cell wall. The genetic material is not in a nucleus but a single loop. 3. Prokaryotic cells can contain plasmids. 4. Prokaryotic cells are much smaller than eukaryotic cells. 5. Animal cells contain nucleus, cytoplasm, cell membrane, mitochondria and ribosomes. 6. Plant cells also contain chloroplasts, a vacuole and a cell wall. 7. Plant cell walls are made of cellulose. 8. Most animal cells differentiate at an early stage (become specialised) 9. Most plant cells retain the ability to differentiate throughout their life. 10. Cells can be specialised to carry out a particular function e.g. sperm cells, nerve cells, muscle cells, root hair cells, xylem and phloem cells. Cells All living things are made of ________________. Cells can either be ____________________ or ________________________. Plants and animal cells are _________________________. Label the diagram of a plant and animal cell. Compare the structure of a plant and animal cell Bacteria are _______________________ cells. Label the diagram of a bacterial cell. Complete the Venn Diagram to compare eukaryotic and prokaryotic cells Eukaryotic Prokaryotic Function of cell organelles Learning List – Microscopes 1. Light microscopes use light and lenses to form an image of a specimen. 2. Light microscopes are used to se nuclei, chloroplasts, cell wall, cell membrane and mitochondria. Electron microscopes use electrons to form an image. 3. Stains are used to make the specimen visible. 4. Electron microscopes have a higher magnification. -

Summary a Plant Is an Integrated System Which: 1
Summary A plant is an integrated system which: 1. Obtains water and nutrients from the soil. 2. Transports them 3. Combines the H2O with CO2 to make sugar. 4. Exports sugar to where it’s needed Today, we’ll start to go over how this occurs Transport in Plants – Outline I.I. PlantPlant waterwater needsneeds II.II. TransportTransport ofof waterwater andand mineralsminerals A.A. FromFrom SoilSoil intointo RootsRoots B.B. FromFrom RootsRoots toto leavesleaves C.C. StomataStomata andand transpirationtranspiration WhyWhy dodo plantsplants needneed soso muchmuch water?water? TheThe importanceimportance ofof waterwater potential,potential, pressure,pressure, solutessolutes andand osmosisosmosis inin movingmoving water…water… Transport in Plants 1.1. AnimalsAnimals havehave circulatorycirculatory systems.systems. 2.2. VascularVascular plantsplants havehave oneone wayway systems.systems. Transport in Plants •• OneOne wayway systems:systems: plantsplants needneed aa lotlot moremore waterwater thanthan samesame sizedsized animals.animals. •• AA sunflowersunflower plantplant “drinks”“drinks” andand “perspires”“perspires” 1717 timestimes asas muchmuch asas aa human,human, perper unitunit ofof mass.mass. Transport of water and minerals in Plants WaterWater isis goodgood forfor plants:plants: 1.1. UsedUsed withwith CO2CO2 inin photosynthesisphotosynthesis toto makemake “food”.“food”. 2.2. TheThe “blood”“blood” ofof plantsplants –– circulationcirculation (used(used toto movemove stuffstuff around).around). 3.3. EvaporativeEvaporative coolingcooling. -

Dissecting Hierarchies Between Light, Sugar and Auxin Action Underpinning Root and Root Hair Growth
plants Article Dissecting Hierarchies between Light, Sugar and Auxin Action Underpinning Root and Root Hair Growth Judith García-González 1,2,†, Jozef Lacek 1,† and Katarzyna Retzer 1,* 1 Laboratory of Hormonal Regulations in Plants, Institute of Experimental Botany, Czech Academy of Sciences, 165 02 Prague, Czech Republic; [email protected] (J.G.-G.); jozefl[email protected] (J.L.) 2 Department of Experimental Plant Biology, Faculty of Science, Charles University, 128 00 Prague, Czech Republic * Correspondence: [email protected] † Those authors contributed equally to this work. Abstract: Plant roots are very plastic and can adjust their tissue organization and cell appearance during abiotic stress responses. Previous studies showed that direct root illumination and sugar supplementation mask root growth phenotypes and traits. Sugar and light signaling where further connected to changes in auxin biosynthesis and distribution along the root. Auxin signaling un- derpins almost all processes involved in the establishment of root traits, including total root length, gravitropic growth, root hair initiation and elongation. Root hair plasticity allows maximized nutrient uptake and therefore plant productivity, and root hair priming and elongation require proper auxin availability. In the presence of sucrose in the growth medium, root hair emergence is partially rescued, but the full potential of root hair elongation is lost. With our work we describe a combinatory study showing to which extent light and sucrose are antagonistically influencing root length, but additively affecting root hair emergence and elongation. Furthermore, we investigated the impact of the loss of PIN-FORMED2, an auxin efflux carrier mediating shootward auxin transporter, on the establishment of root traits in combination with all growth conditions. -

Transport of Water and Solutes in Plants
Transport of Water and Solutes in Plants Water and Solute Potential Water potential is the measure of potential energy in water and drives the movement of water through plants. LEARNING OBJECTIVES Describe the water and solute potential in plants Key Points Plants use water potential to transport water to the leaves so that photosynthesis can take place. Water potential is a measure of the potential energy in water as well as the difference between the potential in a given water sample and pure water. Water potential is represented by the equation Ψsystem = Ψtotal = Ψs + Ψp + Ψg + Ψm. Water always moves from the system with a higher water potential to the system with a lower water potential. Solute potential (Ψs) decreases with increasing solute concentration; a decrease in Ψs causes a decrease in the total water potential. The internal water potential of a plant cell is more negative than pure water; this causes water to move from the soil into plant roots via osmosis.. Key Terms solute potential: (osmotic potential) pressure which needs to be applied to a solution to prevent the inward flow of water across a semipermeable membrane transpiration: the loss of water by evaporation in terrestrial plants, especially through the stomata; accompanied by a corresponding uptake from the roots water potential: the potential energy of water per unit volume; designated by ψ Water Potential Plants are phenomenal hydraulic engineers. Using only the basic laws of physics and the simple manipulation of potential energy, plants can move water to the top of a 116-meter-tall tree. Plants can also use hydraulics to generate enough force to split rocks and buckle sidewalks. -

The Osmotic Adjustment of Marine Microalgae
The osmotic adjustment of marine microalgae and the complex role osmolytes play in this process Thesis of Liz Kendall Supervisor Dr. Gieskes Department of Marine Biology, The University of Groningen April 1996 D5O Summary: Algae inhabit a wide variety of both marine and freshwater habitats. These habitats differ in regard to various factors such as chemical composition, the organisms that live there, the light which may radiate into that particular area, the temperature of the sites depending on where the environment is located, just to name a few. One factor that varies from environment to environment is the salinity. This paper will look at the mechanisms utilized by marine algae to cope with the changes in salinity content in their habitats and most importantly how they use different osmolytes to carry out this process. Marine algae "osmotically adjust" themselves to external salinity changes, in a biphasic maimer. Firstly, this includes changes in turgor pressure or large internal water fluxes in response to osmotic gradients. Secondly. an internally regulated osmotic adjustment occurs with the use of both inorganic and organic osmolytes. Compatible solutes are ions and molecules used by man organisms to osmotically adjust and they play a double role in the process of osmotic adjustment. They act as osmolytes and also protect the cellular enzymatic activities under salinity stress. They are called 'compatible solutes" because they protect the cellular enzymatic activity. The main compatible solutes are polyols (including amino acids, carbohydrates and sugars). quaternary ammonium derivatives or tertiary sulphonium compounds. Certain species and taxonomic classes use specific compatible solutes and some even use combinations of them. -
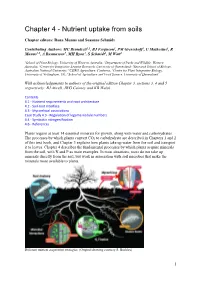
Nutrient Uptake from Soils
Chapter 4 - Nutrient uptake from soils Chapter editors: Rana Munns and Susanne Schmidt Contributing Authors: MC Brundrett1,2, BJ Ferguson3, PM Gressshoff3, U Mathesius5, R Munns1,6, A Rasmussen7, MH Ryan1, S Schmidt8, M Watt5 1School of Plant Biology, University of Western Australia; 2Department of Parks and Wildlife, Western Australia; 3Centre for Integrative Legume Research, University of Queensland; 5Research School of Biology, Australian National University; 6CSIRO Agriculture, Canberra; 7Centre for Plant Integrative Biology, University of Nottingham, UK; 8School of Agriculture and Food Science, University of Queensland With acknowledgements to authors of the original edition Chapter 3, sections 3, 4 and 5 respectively: BJ Atwell, JWG Cairney and KB Walsh Contents 4.1 - Nutrient requirements and root architecture 4.2 - Soil-root interface 4.3 - Mycorrhizal associations Case Study 4.3 - Regulation of legume nodule numbers 4.4 - Symbiotic nitrogen fixation 4.6 - References Plants require at least 14 essential minerals for growth, along with water and carbohydrates. The processes by which plants convert CO2 to carbohydrate are described in Chapters 1 and 2 of this text book, and Chapter 3 explains how plants take up water from the soil and transport it to leaves. Chapter 4 describes the fundamental processes by which plants acquire minerals from the soil, with N and P as main examples. In most situations, roots do not take up minerals directly from the soil, but work in association with soil microbes that make the minerals more available to plants. Different nutrient acquisition strategies. (Original drawing courtesy S. Buckley) 1 This chapter first explains the concept of plant nutrition, then describes the various root structures and symbioses with microorganisms that allow plants to take up essential nutrients. -

Historical Review
1 Historical Review INTRODUCTION This chapter presents a brief historical review of progress in the field of plant water relations because the authors feel that it is impossible to fully understand the present without some knowledge of the past. As the Danish philosopher Kierkegaarde wrote, "Life can only be understood backward, but it can only be lived forward," and this also is true of science. The present generation needs to be reminded that some generally accepted concepts have their origin in ideas of 17th or 18th century writers and although others were suggested many decades ago, they were neglected until recently. As might be expected, the importance of water to plant growth was recog- nized by prehistoric farmers because irrigation systems already existed in Egypt, Babylonia (modern Iraq), and China at the beginning of recorded history, and the first European explorers found extensive irrigation systems in both North and South America. However, irrigation was not used extensively in agriculture in the United States until after the middle of the 19th century and little research on plant water relations occurred until the 20th century. Early Research Although plant water relations appear to have been the first area of plant physiology to be studied, progress was slow from Aristotle who died in 322 B.C. to the middle of the 19th century. According to Aristotle, plants absorbed their food ready for use from the soil, and plant nutrition was controlled by a soul or vital principle that ailowed plants to absorb only those substances useful in 2 1. Historical Review growth. This idea only began to be questioned in the 17th century by Jung, van Helmont, Mariotte, and others, and it ~ersistedinto the 19th century. -

Fine Structure of Root Hair Infection Leading to Nodulation in the Frankia -Ainus Symbiosis
Fine structure of root hair infection leading to nodulation in the Frankia -Ainus symbiosis A. M. BERRY Department of Environmental Horticulture, University of California, Davis, CA, U.S.A. 9561 6 AND L. MCINTYREAND M. E. MCCULLY Biology Department, Carleton University, Ottawa, Ont., Canada KlS 3B6 Received January 24, 1985 BERRY,A. M., L. MCINTYRE,and M. E. MCCULLY.1986. Fine structure of root hair infection leading to nodulation in the Frankia - Alnus symbiosis. Can. J. Bot. 64: 292 -305. Root hair infection by Frankia (Actinomycetales) is the means by which nitrogen-fixing root nodules are initiated upon the actinorhizal host, Alnus rubra. Structural details of the infectious process and the changes in host root hair cells are demonstrated at the prenodule stage for the first time using light and transmission electron microscopy. The Frankia hypha is the infective agent, extending from the rhizosphere through the root hair wall in a highly deformed region of the hair. There is no evidence of pleomorphism of the Frankia hypha. The primary wall fibrils of the root hair appear disorganized at the site of penetration. There is extensive secondary wall formation in the infected hair. At the site of penetration, root hair cell wall ingrowths occur that are structurally consistent with transfer cell wall formation. The ingrowths are continuous with the encapsulating wall layer surrounding the Frankia hypha The host cytoplasm is rich in ribosomes, secretory products, and organelles, including Golgi bodies, mitochondria, plastids, and profiles of endoplasmic reticulum. In an aborted infection sequence, some structural features of the host response to Frankia are observable, while other aspects of successful infection do not occur. -

Unleashing the Potential of the Root Hair Cell As a Single Plant Cell Type Model in Root Systems Biology
METHODS ARTICLE published: 26 November 2013 doi: 10.3389/fpls.2013.00484 Unleashing the potential of the root hair cell as a single plant cell type model in root systems biology Zhenzhen Qiao and Marc Libault* Department of Microbiology and Plant Biology, University of Oklahoma, Norman, OK, USA Edited by: Plant root is an organ composed of multiple cell types with different functions. This Wolfgang Schmidt, Academia Sinica, multicellular complexity limits our understanding of root biology because -omics studies Taiwan performed at the level of the entire root reflect the average responses of all cells composing Reviewed by: the organ. To overcome this difficulty and allow a more comprehensive understanding of Georgina Hernandez, Universidad Autónoma de Mexico, Mexico root cell biology, an approach is needed that would focus on one single cell type in the Jeremy Dale Murray, John Innes plant root. Because of its biological functions (i.e., uptake of water and various nutrients; Centre, UK primary site of infection by nitrogen-fixing bacteria in legumes), the root hair cell is an *Correspondence: attractive single cell model to study root cell response to various stresses and treatments. Marc Libault, Department of To fully study their biology, we have recently optimized procedures in obtaining root hair Microbiology and Plant Biology, University of Oklahoma, 770 Van Vleet cell samples. We culture the plants using an ultrasound aeroponic system maximizing root Oval, Norman, OK 73019, USA hair cell density on the entire root systems and allowing the homogeneous treatment of e-mail: [email protected] the root system. We then isolate the root hair cells in liquid nitrogen.