Looking-For-Solutions-To-The-Pitfalls-Of
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Infectious Diseases
2013 MEDICINES IN DEVELOPMENT REPORT Infectious Diseases A Report on Diseases Caused by Bacteria, Viruses, Fungi and Parasites PRESENTED BY AMERICA’S BIOPHARMACEUTICAL RESEARCH COMPANIES Biopharmaceutical Research Evolves Against Infectious Diseases with Nearly 400 Medicines and Vaccines in Testing Throughout history, infectious diseases hepatitis C that inhibits the enzyme have taken a devastating toll on the lives essential for viral replication. and well-being of people around the • An anti-malarial drug that has shown Medicines in Development world. Caused when pathogens such activity against Plasmodium falci- For Infectious Diseases as bacteria or viruses enter a body and parum malaria which is resistant to multiply, infectious diseases were the current treatments. Application leading cause of death in the United Submitted States until the 1920s. Today, vaccines • A potential new antibiotic to treat methicillin-resistant Staphylococcus Phase III and infectious disease treatments have proven to be effective treatments in aureus (MRSA). Phase II many cases, but infectious diseases still • A novel treatment that works by Phase I pose a very serious threat to patients. blocking the ability of the smallpox Recently, some infectious pathogens, virus to spread to other cells, thus 226 such as pseudomonas bacteria, have preventing it from causing disease. become resistant to available treatments. Infectious diseases may never be fully Diseases once considered conquered, eradicated. However, new knowledge, such as tuberculosis, have reemerged new technologies, and the continuing as a growing health threat. commitment of America’s biopharma- America’s biopharmaceutical research ceutical research companies can help companies are developing 394 medicines meet the continuing—and ever-changing and vaccines to combat the many threats —threat from infectious diseases. -

NASDAQ Stock Market
Nasdaq Stock Market Friday, December 28, 2018 Name Symbol Close 1st Constitution Bancorp FCCY 19.75 1st Source SRCE 40.25 2U TWOU 48.31 21st Century Fox Cl A FOXA 47.97 21st Century Fox Cl B FOX 47.62 21Vianet Group ADR VNET 8.63 51job ADR JOBS 61.7 111 ADR YI 6.05 360 Finance ADR QFIN 15.74 1347 Property Insurance Holdings PIH 4.05 1-800-FLOWERS.COM Cl A FLWS 11.92 AAON AAON 34.85 Abiomed ABMD 318.17 Acacia Communications ACIA 37.69 Acacia Research - Acacia ACTG 3 Technologies Acadia Healthcare ACHC 25.56 ACADIA Pharmaceuticals ACAD 15.65 Acceleron Pharma XLRN 44.13 Access National ANCX 21.31 Accuray ARAY 3.45 AcelRx Pharmaceuticals ACRX 2.34 Aceto ACET 0.82 Achaogen AKAO 1.31 Achillion Pharmaceuticals ACHN 1.48 AC Immune ACIU 9.78 ACI Worldwide ACIW 27.25 Aclaris Therapeutics ACRS 7.31 ACM Research Cl A ACMR 10.47 Acorda Therapeutics ACOR 14.98 Activision Blizzard ATVI 46.8 Adamas Pharmaceuticals ADMS 8.45 Adaptimmune Therapeutics ADR ADAP 5.15 Addus HomeCare ADUS 67.27 ADDvantage Technologies Group AEY 1.43 Adobe ADBE 223.13 Adtran ADTN 10.82 Aduro Biotech ADRO 2.65 Advanced Emissions Solutions ADES 10.07 Advanced Energy Industries AEIS 42.71 Advanced Micro Devices AMD 17.82 Advaxis ADXS 0.19 Adverum Biotechnologies ADVM 3.2 Aegion AEGN 16.24 Aeglea BioTherapeutics AGLE 7.67 Aemetis AMTX 0.57 Aerie Pharmaceuticals AERI 35.52 AeroVironment AVAV 67.57 Aevi Genomic Medicine GNMX 0.67 Affimed AFMD 3.11 Agile Therapeutics AGRX 0.61 Agilysys AGYS 14.59 Agios Pharmaceuticals AGIO 45.3 AGNC Investment AGNC 17.73 AgroFresh Solutions AGFS 3.85 -

P39262 Alimera Sciences, Inc. Nps 2020 V1
Alimera Sciences, Inc. 6120 Windward Parkway, Suite 290 Alpharetta, Georgia 30005 NOTICE OF ANNUAL MEETING OF STOCKHOLDERS TO BE HELD ON JUNE 18, 2020 To the Stockholders of Alimera Sciences, Inc.: The annual meeting of stockholders (the “Annual Meeting”) of Alimera Sciences, Inc. (the “Company”) will be held exclusively online via the Internet on Thursday, June 18, 2020, at 9:30 a.m. Eastern Time. The purposes of the meeting are: 1. To elect three Class I directors (Proposal 1); 2. To ratify the appointment of Grant Thornton LLP as our independent registered public accounting firm for the fiscal year ending December 31, 2020 (Proposal 2); 3. To approve, on an advisory basis, the compensation of our named executive officers (Proposal 3); and 4. To transact such other business as may properly come before the Annual Meeting or any adjournments or postponements thereof. Our board of directors (the “Board”) has fixed the close of business on April 20, 2020 as the record date (the “record date”) for determining holders of our common stock and preferred stock entitled to notice of, and to vote at, the Annual Meeting or any adjournments or postponements thereof. This year we are again using the Internet as our primary means of furnishing proxy materials to stockholders. Accordingly, most stockholders will not receive printed copies of our proxy materials. We are instead mailing a Notice of Internet Availability of Proxy Materials with instructions for accessing the proxy materials and voting via the Internet (the “Notice”). This delivery method allows us to conserve natural resources and reduce the cost of delivery while also meeting our obligations to you, our stockholders, to provide information relevant to your continued investment in the Company. -

Rebateable Manufacturers
Rebateable Labelers – July 2021 Manufacturers are responsible for updating their eligible drugs and pricing with CMS. Montana Healthcare Programs will not pay for an NDC not updated with CMS. Note: Some manufacturers on this list may have some NDCs that are covered and others that are not. Manufacturer ID Manufacturer Name 00002 ELI LILLY AND COMPANY 00003 E.R. SQUIBB & SONS, LLC. 00004 HOFFMANN-LA ROCHE 00006 MERCK & CO., INC. 00007 GLAXOSMITHKLINE 00008 WYETH PHARMACEUTICALS LLC, 00009 PHARMACIA AND UPJOHN COMPANY LLC 00013 PFIZER LABORATORIES DIV PFIZER INC 00015 MEAD JOHNSON AND COMPANY 00023 ALLERGAN INC 00024 SANOFI-AVENTIS, US LLC 00025 PFIZER LABORATORIES DIV PFIZER INC 00026 BAYER HEALTHCARE LLC 00032 ABBVIE INC. 00037 MEDA PHARMACEUTICALS, INC. 00039 SANOFI-AVENTIS, US LLC 00046 WYETH PHARMACEUTICALS INC. 00049 ROERIG 00051 ABBVIE INC 00052 ORGANON USA INC. 00053 CSL BEHRING L.L.C. 00054 HIKMA PHARMACEUTICAL USA, INC. 00056 BRISTOL-MYERS SQUIBB PHARMA CO. 00065 ALCON LABORATORIES, INC. 00068 AVENTIS PHARMACEUTICALS 00069 PFIZER LABORATORIES DIV PFIZER INC 00071 PARKE-DAVIS DIV OF PFIZER 00074 ABBVIE INC 00075 AVENTIS PHARMACEUTICALS, INC. 00078 NOVARTIS 00085 SCHERING CORPORATION 00087 BRISTOL-MYERS SQUIBB COMPANY 00088 AVENTIS PHARMACEUTICALS 00093 TEVA PHARMACEUTICALS USA, INC. 00095 BAUSCH HEALTH US, LLC Page 1 of 19 Manufacturer ID Manufacturer Name 00096 PERSON & COVEY, INC. 00113 L. PERRIGO COMPANY 00115 IMPAX GENERICS 00116 XTTRIUM LABORATORIES, INC. 00121 PHARMACEUTICAL ASSOCIATES, INC. 00131 UCB, INC. 00132 C B FLEET COMPANY INC 00143 HIKMA PHARMACEUTICAL USA, INC. 00145 STIEFEL LABORATORIES, INC, 00168 E FOUGERA AND CO. 00169 NOVO NORDISK, INC. 00172 TEVA PHARMACEUTICALS USA, INC 00173 GLAXOSMITHKLINE 00178 MISSION PHARMACAL COMPANY 00185 EON LABS, INC. -

Emmett Cunningham, Jr., M.D., Ph.D., M.P.H
2019 - Emmett Cunningham, Jr., M.D., Ph.D., M.P.H. Senior Managing Director Blackstone Life Sciences HEATHEGY TEAM CRAIG SIMAK CRAIG SIMAK DANIELLE SILVA MAUREEN LINNEMANN 1200 OIS@AAO 24 Meetings > 1,150 1000 ~ 13,500 Attendees 800 OIS@ASCRS 600 > 650 400 OIS@ASRS 200 > 300 0 2009 2010 2011 2012 2013 2014 2015 2016 2017 2018 2019 2020 Registrants 3% 10% OIS@AAO 2019 ( > 1,150) 8% Academia, Government, or Association 41% Adviser, Consultant, or Service Provider Finance/Investment Firm Large Multi-National Corporation Physician/Healthcare Provider 25% Press/Media Start-up/Emerging Growth Company 3% 25 Countries 10% 32 US States Record Number of CDER NME NDA/BLA Approvals in 2018 70 BLA 59 60 NDA 50 45 46 Number 39 41 of 40 35 36 Drugs 30 30 30 27 27 24 26 22 24 21 20 21 22 20 17 18 10 0 1998 1999 2000 2001 2002 2003 2004 2005 2006 2007 2008 2009 2010 2011 2012 2013 2014 2015 2016 2017 2018 Source: FDA 1998 - 2018 High Innovation Record Number of • 31 (53%) Orphan CDER NME NDA/BLA Approvals in 2018 • 26 (44%) Priority Review • 16 (27%) Fast Track • 12 (20%) Break Through Designation 59 • 18 (30%) Oncology • 1 (0%) Ophthalmology Cenegermin-bkbj Ophthalmic Solution, (OXERVATE®) Ophthalmic Drugs Streamlined Reporting of Ophthalmology Clinical Group CDER As of January, 2018 Ophthalmology Clinical Group Reported Directly & Independently to Deputy Director Office of New Drugs Peter Stein, MD Five Additional Office of Offices of Drug Antimicrobial Evaluation (ODEs) Products Division of Division of Division of Transplant and Anti-Infective Anti-Viral Ophthalmology Products Products Dr. -

57Thannual ASSEMBLY
PHARMACISTS AS PROVIDERS: COMPLETING THE CIRCLE OF PATIENT-CENTERED CARE. 57th ANNUAL ASSEMBLY ANNUAL PROGRAM APRIL 19-22, 2018 SARATOGA SPRINGS CITY CENTER - SARATOGA SPRINGS, NY MESSAGE FROM PRESIDENT Dear Colleagues, I am excited to invite you to the NYSCHP 57th Annual Assembly at The Saratoga Springs City Center in Saratoga Springs, NY from Thursday, April 19 to Sunday, April 22, 2018. The NYSCHP Annual Assembly is the premier New York meeting of Health-system pharmacists from around the State who gather to learn from pharmacy leaders, discuss challenges and best practices, build Professional networks, and plan for future practice improvements. The Annual Assembly typically draws over 350 attendees from student – pharmacists to Chief Pharmacy Officers and administrators. It is the single most important meeting for New York State Health-system Pharmacists to attend each year. The Annual Assembly typically draws over 80 industry exhibitors. Our industry colleagues and partners have the opportunity to display, demonstrate, and discuss their products and services. The exhibit hall will take place on Friday, April 20, providing two hours for conference attendees to meet with exhibitors and discuss the latest information about pharmaceuticals, technologies and products. In addition, two Director of Pharmacy/Industry networking event sessions are offered: a 90-minute session on Thursday immediately following the first House of Delegates meeting, and a 75-minute session on Saturday, just prior to the Installation Banquet. The Director of Pharmacy/ Industry networking sessions provide an exceptional opportunity to discuss your products and solutions and build long-lasting relationships. We are particularly proud that we will once again feature the Residency Research & Practice Forum in conjunction with the Annual Assembly; residents from around the state will be performing platform presentations on their residency projects. -

Manufacturers and Wholesalers Street City ST Zip Abbott Diabetes Care Division Abbott Diagnostic Division Abbott Laboratories 100 Abbott Park Road, Dept
2018 AB128 Code of Conduct Compliant Companies report Manufacturers and Wholesalers Street City ST Zip Abbott Diabetes Care Division Abbott Diagnostic Division Abbott Laboratories 100 Abbott Park Road, Dept. EC10, Bldg. APGA-2 Abbott Park IL 60064 Abbott Molecular Division Abbott Nutrition Products Division Abbott Vascular Division AbbVie, Inc. 1 N. Waukegan Road North Chicago IL 60064 Acadia Phamaceuticals 3611 Valley Centre Drive, Suite 300 San Diego CA 92130 Accelero Health Partners, LLC Acclarent, Inc. 1525-B O'Brien Dr. Menlo Park CA 94025 Accutron Inc. Ace Surgical Supply, Inc. 1034 Pearl St. Brockton MA 02301 Acell, Inc. 6640 Eli Whitney Drive, Suite 200 Columbia MD 21046 Aclaris Therapeutics, Inc. 640 Lee Road, Suite 200 Wayne PA 19087 Acorda Therapeutics, Inc. 420 Sawmill River Road Ardsley NY 10532 AcriVet Inc. Actelion Pharmaceuticals US, Inc. 5000 Shoreline Court, Suite 200 S. San Francisco CA 94080 Adamas Pharmaceuticals, Inc. 1900 Powell St., Suite 750 Emeryville CA 94608 A-Dec, Inc. 2601 Crestview Dr. Newberg OR 97132 Advanced Respiratory, Inc. Advanced Sterilization Products Services, Inc. Aegerion Pharmaceuticals, Inc. One Main Street, Suite 800 Cambridge MA 02142 Aerie Pharmaceuticals, Inc. 2030 Main St., Suite 1400 Irvine CA 92614 Aesculap Implant Systems, Inc. Aesculap, Inc. 3773 Corporate Parkway Center Valley PA 18034 Aesthera Corporation Afaxys, Inc. PO Box 20158 Charleston SC 29413 AGMS Inc. Akrimax Pharmaceuticals, LLC 11 Commerce Drive, 1st Floor Cranford NJ 07016 Alamo Pharma Services, Inc. Alcon Laboratories, Inc. 6201 South Freeway Fort Worth TX 76134 Alden Optical Laboratories, Inc. Aldenex Vision LLC Alimera Sciences, Inc. 6120 Windward Parkway, Suite 290 Alpharetta GA 30005 Alkermes, Inc. -

Ligand Partner Melinta Therapeutics Reports Positive Phase 3 Results for Captisol-Enabled™ Delafloxacin IV
January 8, 2015 Ligand Partner Melinta Therapeutics Reports Positive Phase 3 Results for Captisol-enabled™ Delafloxacin IV SAN DIEGO-- Ligand Pharmaceuticals Incorporated (NASDAQ: LGND) announced today that its partner Melinta Therapeutics reported positive top-line results from the first of two Phase 3 PROCEED studies (study RX-3341-302, NCT01811732) to evaluate delafloxacin, an investigational fluoroquinolone, compared with vancomycin + aztreonam for the treatment of patients with acute bacterial skin and skin structure infections (ABSSSI). Delafloxacin met the study’s primary objective endpoint of a reduction in the measurement of lesion erythema at the primary infection site at 48- to- 72 hours, the endpoint required by the U.S. Food and Drug Administration (FDA). Delafloxacin also was comparable to vancomycin in the study’s secondary endpoints, including investigator assessment of signs and symptoms of infection at the follow-up visit, a metric required by the European Medicines Agency (EMA). “Our partners at Melinta Therapeutics continue to make great progress advancing Captisol- enabled Delafloxacin IV,” said John Higgins, Ligand’s President and Chief Executive Officer. “We congratulate them on these positive results as they move delafloxacin toward the market to treat serious, often life-threatening infections. Captisol-enabled drugs continue to show great promise in a variety of therapy areas that have major global unmet medical need, including infectious diseases, oncology, CNS, emergency care and others.” Melinta reports that delafloxacin was shown to be well tolerated among study participants. Mild gastrointestinal symptoms were the most common treatment-related adverse events reported for delafloxacin, and rarely led to study discontinuation. Melinta expects to make complete results from this study available as a presentation at an upcoming medical meeting and a publication in a peer-reviewed medical journal. -
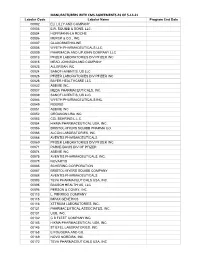
MANUFACTURERS with CMS AGREEMENTS AS of 5-14-21 Labeler Code Labeler Name Program End Date 00002 ELI LILLY and COMPANY 00003 E.R
MANUFACTURERS WITH CMS AGREEMENTS AS OF 5-14-21 Labeler Code Labeler Name Program End Date 00002 ELI LILLY AND COMPANY 00003 E.R. SQUIBB & SONS, LLC. 00004 HOFFMANN-LA ROCHE 00006 MERCK & CO., INC. 00007 GLAXOSMITHKLINE 00008 WYETH PHARMACEUTICALS LLC, 00009 PHARMACIA AND UPJOHN COMPANY LLC 00013 PFIZER LABORATORIES DIV PFIZER INC 00015 MEAD JOHNSON AND COMPANY 00023 ALLERGAN INC 00024 SANOFI-AVENTIS, US LLC 00025 PFIZER LABORATORIES DIV PFIZER INC 00026 BAYER HEALTHCARE LLC 00032 ABBVIE INC. 00037 MEDA PHARMACEUTICALS, INC. 00039 SANOFI-AVENTIS, US LLC 00046 WYETH PHARMACEUTICALS INC. 00049 ROERIG 00051 ABBVIE INC 00052 ORGANON USA INC. 00053 CSL BEHRING L.L.C. 00054 HIKMA PHARMACEUTICAL USA, INC. 00056 BRISTOL-MYERS SQUIBB PHARMA CO. 00065 ALCON LABORATORIES, INC. 00068 AVENTIS PHARMACEUTICALS 00069 PFIZER LABORATORIES DIV PFIZER INC 00071 PARKE-DAVIS DIV OF PFIZER 00074 ABBVIE INC 00075 AVENTIS PHARMACEUTICALS, INC. 00078 NOVARTIS 00085 SCHERING CORPORATION 00087 BRISTOL-MYERS SQUIBB COMPANY 00088 AVENTIS PHARMACEUTICALS 00093 TEVA PHARMACEUTICALS USA, INC. 00095 BAUSCH HEALTH US, LLC 00096 PERSON & COVEY, INC. 00113 L. PERRIGO COMPANY 00115 IMPAX GENERICS 00116 XTTRIUM LABORATORIES, INC. 00121 PHARMACEUTICAL ASSOCIATES, INC. 00131 UCB, INC. 00132 C B FLEET COMPANY INC 00143 HIKMA PHARMACEUTICAL USA, INC. 00145 STIEFEL LABORATORIES, INC, 00168 E FOUGERA AND CO. 00169 NOVO NORDISK, INC. 00172 TEVA PHARMACEUTICALS USA, INC Labeler Code Labeler Name Program End Date 00173 GLAXOSMITHKLINE 00178 MISSION PHARMACAL COMPANY 00185 EON LABS, INC. 00186 ASTRAZENECA PHARMACEUTICALS LP 00187 BAUSCH HEALTH US, LLC. 00206 WYETH PHARMACEUTICALS LLC, 00224 KONSYL PHARMACEUTICALS, INC. 00225 B. F. ASCHER AND COMPANY, INC. 00228 ACTAVIS PHARMA, INC. -

SCHEDULE 14A Trevena, Inc
UNITED STATES SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 SCHEDULE 14A Proxy Statement Pursuant to Section 14(a) of the Securities Exchange Act of 1934 (Amendment No. ) Filed by the Registrant x Filed by a Party other than the Registrant ¨ Check the appropriate box: x Preliminary Proxy Statement ¨ Confidential, for Use of the Commission Only (as permitted by Rule 14a-6(e)(2)) ¨ Definitive Proxy Statement ¨ Definitive Additional Materials ¨ Soliciting Material under §240.14a-12 Trevena, Inc. (Name of Registrant as Specified in Its Charter) (Name of Person(s) Filing Proxy Statement, if other than the Registrant) Payment of Filing Fee (Check the appropriate box): x No fee required. ¨ Fee computed on table below per Exchange Act Rules 14a-6(i)(1) and 0-11. (1) Title of each class of securities to which transaction applies: (2) Aggregate number of securities to which transaction applies: (3) Per unit price or other underlying value of transaction computed pursuant to Exchange Act Rule 0-11 (set forth the amount on which the filing fee is calculated and state how it was determined): (4) Proposed maximum aggregate value of transaction: (5) Total fee paid: ¨ Fee paid previously with preliminary materials. ¨ Check box if any part of the fee is offset as provided by Exchange Act Rule 0-11(a)(2) and identify the filing for which the offsetting fee was paid previously. Identify the previous filing by registration statement number, or the Form or Schedule and the date of its filing. (1) Amount Previously Paid: (2) Form, Schedule or Registration Statement No.: (3) Filing Party: (4) Date Filed: April 13, 2020 955 Chesterbrook Boulevard, Suite 110 Chesterbrook, PA 19087 Dear Trevena Stockholder: On behalf of the Trevena, Inc. -

Appendix B - Product Name Sorted by Applicant
JUNE 2021 - APPROVED DRUG PRODUCT LIST B - 1 APPENDIX B - PRODUCT NAME SORTED BY APPLICANT ** 3 ** 3D IMAGING DRUG * 3D IMAGING DRUG DESIGN AND DEVELOPMENT LLC AMMONIA N 13, AMMONIA N-13 FLUDEOXYGLUCOSE F18, FLUDEOXYGLUCOSE F-18 SODIUM FLUORIDE F-18, SODIUM FLUORIDE F-18 3M * 3M CO PERIDEX, CHLORHEXIDINE GLUCONATE * 3M HEALTH CARE INC AVAGARD, ALCOHOL (OTC) DURAPREP, IODINE POVACRYLEX (OTC) 3M HEALTH CARE * 3M HEALTH CARE INFECTION PREVENTION DIV SOLUPREP, CHLORHEXIDINE GLUCONATE (OTC) ** 6 ** 60 DEGREES PHARMS * 60 DEGREES PHARMACEUTICALS LLC ARAKODA, TAFENOQUINE SUCCINATE ** A ** AAA USA INC * ADVANCED ACCELERATOR APPLICATIONS USA INC LUTATHERA, LUTETIUM DOTATATE LU-177 NETSPOT, GALLIUM DOTATATE GA-68 AAIPHARMA LLC * AAIPHARMA LLC AZASAN, AZATHIOPRINE ABBVIE * ABBVIE INC ANDROGEL, TESTOSTERONE CYCLOSPORINE, CYCLOSPORINE DEPAKOTE ER, DIVALPROEX SODIUM DEPAKOTE, DIVALPROEX SODIUM GENGRAF, CYCLOSPORINE K-TAB, POTASSIUM CHLORIDE KALETRA, LOPINAVIR NIASPAN, NIACIN NIMBEX PRESERVATIVE FREE, CISATRACURIUM BESYLATE NIMBEX, CISATRACURIUM BESYLATE NORVIR, RITONAVIR SYNTHROID, LEVOTHYROXINE SODIUM ** TARKA, TRANDOLAPRIL TRICOR, FENOFIBRATE TRILIPIX, CHOLINE FENOFIBRATE ULTANE, SEVOFLURANE ZEMPLAR, PARICALCITOL ABBVIE ENDOCRINE * ABBVIE ENDOCRINE INC LUPANETA PACK, LEUPROLIDE ACETATE ABBVIE ENDOCRINE INC * ABBVIE ENDOCRINE INC LUPRON DEPOT, LEUPROLIDE ACETATE LUPRON DEPOT-PED KIT, LEUPROLIDE ACETATE ABBVIE INC * ABBVIE INC DUOPA, CARBIDOPA MAVYRET, GLECAPREVIR NORVIR, RITONAVIR ORIAHNN (COPACKAGED), ELAGOLIX SODIUM,ESTRADIOL,NORETHINDRONE ACETATE -

Melinta and Cempra Announce Accomplished Executive Leadership Team for Combined Organization
Melinta and Cempra Announce Accomplished Executive Leadership Team for Combined Organization NEW HAVEN, Conn. and CHAPEL HILL, N.C., Nov. 01, 2017 (GLOBE NEWSWIRE) -- Melinta Therapeutics, Inc., a privately held company developing and commercializing novel antibiotics to treat serious bacterial infections, and Cempra, Inc. (Nasdaq: CEMP) today announced the executive leadership team for the combined Melinta and Cempra business, which will keep the name Melinta Therapeutics upon the closing of the merger. As previously announced, Dan Wechsler will lead the senior management team of the combined company as president and chief executive officer (CEO). He will be joined by an executive management team with a proven track record of successful execution, including: Executive Officers • Sue Cammarata, MD, Chief Medical Officer Dr. Cammarata, current chief medical officer of Melinta, has more than 20 years of clinical experience in the development, approval and launch of pharmaceuticals, including several anti-infective brands such as Cubicin (daptomycin) in the EU, and Zyvox (linezolid) globally. Since joining Melinta in 2014, she has led the successful Phase 3 clinical development of Baxdela, which was approved by FDA in June, and is also responsible for medical affairs. Prior to joining Melinta, Dr. Cammarata served as vice president of clinical research at Shire HGT, where she was responsible for clinical development and post approval commitments for novel therapies in rare and orphan diseases. Earlier, she held several senior positions at Novartis, most recently as vice president and global program head for the company’s immunology and infectious disease franchises. In this role, she managed the integration of Chiron’s infectious disease portfolio after its acquisition by Novartis in 2006.