Fluoride in Water in Parts of Raniganj Coalfield, West Bengal
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Financial Year
GITANJALI GEMS LIMITED Statement Showing Unpaid / Unclaimed Dividend as on Annual General Meeting held on September 28, 2012 for the financial year 2011‐12 First Name Last Name Address Country State District PINCode Folio Number Investment Type Amount Proposed Date of Securities Due(in of transfer to Rs.) IEPF (DD‐MON‐ YYYY) JYOTSANA OPP SOMESHWAR PART 3 NEAR GULAB TOWER THALTEJ AHMEDABAD INDIA GUJARAT AHMEDABAD 380054 GGL0038799 Amount for unclaimed and unpaid dividend 105.00 08‐OCT‐2019 MANISH BRAHMBHAT 16 MADHUVAN BUNGLOW UTKHANTHESWAR MAHADEV RD AT DEGHAM DIST GANDHINAGAR DEHGAM INDIA GUJARAT GANDHI NAGAR 382305 GGL0124586 Amount for unclaimed and unpaid dividend 105.00 08‐OCT‐2019 BHARAT PATEL A‐8 SHIV PARK SOC NR RAMROY NAGAR N H NO 8 AHMEDABAD INDIA GUJARAT GANDHI NAGAR 382415 GGL0041816 Amount for unclaimed and unpaid dividend 105.00 08‐OCT‐2019 SHARMISTA GANDHI 13 SURYADARSHAN SOC KARELIBAUG VADODARA INDIA GUJARAT VADODARA 390228 GGL0048293 Amount for unclaimed and unpaid dividend 105.00 08‐OCT‐2019 C MALPANI SURAT SURAT INDIA GUJARAT SURAT 395002 GGL0049550 Amount for unclaimed and unpaid dividend 105.00 08‐OCT‐2019 SONAL SHETH C/O CENTURION BANK CENTRAL BOMBAY INFOTECH PARK GR FLR 101 K KHADEVE MARG MAHALAXMI MUMBAI INDIA MAHARASHTRA MUMBAI 400011 GGL0057531 Amount for unclaimed and unpaid dividend 105.00 08‐OCT‐2019 CHIRAG SHAH C/O CENTURION BNK CENTRAL BOMY INFOTECH PARK GR FLR 101 KHADVE MAWRG MAHALAXMI MUMBAI INDIA MAHARASHTRA MUMBAI 400011 GGL0057921 Amount for unclaimed and unpaid dividend 105.00 08‐OCT‐2019 NUPUR C/O -

Active Containment Zone As on 02-10-2020 District: Purulia
District: Purulia Active Containment Zone as on 02-10-2020 Sl. Block/ Operative Police Station Details of Containment Zone No. Municipality Upto 1 Hura Hura House of Magaram Mudi of village Lalpur of M/Lalpur G.P (Magaram Mudi himself is corona positive) 02.10.2020 2 PUNCHA PUNCHA House of Ardhendu Sekhar Gorai,Vill- Pashim Puncha, P.O.+P.S.- Puncha, Dist.- Purulia 02.10.2020 3 Balarampur Balarampur House of Animesh Agarwal, Balarampur. 02-10-2020 4 Balarampur Balarampur House of Maitri Porel, Balarampur 02-10-2020 5 Balarampur Balarampur House of Ashif Ansari at Balarampur Bus Stand 02-10-2020 6 Barabazar Barabazar HOUSE OF SHYAMAPADA SING SARDAR ,AGAJHORE , BARABAZAR 02.10.2020 7 PARA PARA House of Dilip Das at Bauri Para,Village: Fusrabaid,PO:Fusarabaid,Pin Code:723126,under JJ-II GP 02.10.2020 8 Neturia Neturia The house of Naba Bauri, Digha 02.10.2020 9 Neturia Neturia The house of Bipul Maikap, Digha 02.10.2020 10 Arsha Arsha House of BANESWAR MAHATO at JHUNJKA under Hensla G.P. 02.10.2020 11 Arsha Arsha House of AJIT MAHATO at Kashidih under Hetgugui G.P. 02.10.2020 12 Arsha Arsha House of PIRU MAHATO at Chitidih under Mankiary G.P. 02.10.2020 13 Arsha Arsha House of AMBARISH KUMAR at Fosko under Arsha G.P. 02.10.2020 House of Jagabandhu Sarkar, Renter's Name( Covid Positive)- Anirban Haldar,Address- Pahartali, 14 Raghunathpur-I Raghunathpur 02.10.2020 Raghunathpur Word No. 09.PS- Raghunathpur, Dist-Purulia, PIN-723133 House of Prabodh Kr. -

DEVELOPMENT of PUBLIC LIBRARIES in the DISTRICT of PURULIA: a STUDY DEBDAS MONDAL [email protected]
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Library Philosophy and Practice (e-journal) Libraries at University of Nebraska-Lincoln Summer 5-10-2019 DEVELOPMENT OF PUBLIC LIBRARIES IN THE DISTRICT OF PURULIA: A STUDY DEBDAS MONDAL [email protected] Follow this and additional works at: https://digitalcommons.unl.edu/libphilprac Part of the Library and Information Science Commons MONDAL, DEBDAS, "DEVELOPMENT OF PUBLIC LIBRARIES IN THE DISTRICT OF PURULIA: A STUDY" (2019). Library Philosophy and Practice (e-journal). 2740. https://digitalcommons.unl.edu/libphilprac/2740 DEVELOPMENT OF PUBLIC LIBRARIES IN THE DISTRICT OF PURULIA: A STUDY Debdas Mondal Librarian, D.A.V Model School, I.I.T Kharagpur,W.B. [email protected] Kartik Chandra Das Librarian,D.A.V Public School,Haldia [email protected] Abstract The scope of the present review is to cogitate the Public Library scenario in the district of purulia, W.B. It also would reflect their location according to their year of set up and year of sponsorship. The allocation is shown Sub-div, block, Municipal area and Panchayat area wise. The study also focuses the Public Library movement in Purulia district with a conclusion about the necessity of setting up of a public library and recruiting librarians for a well informed society. Keywords: Public Library, Development of Public Library, Purulia District. 1. Introduction In the present era public libraries are the basic units which can provide for the collection of information much needed by the local community where they are set up. This will serve as a gateway of knowledge and information and will enhance opportunity for lifelong learning for the community, which will further help in independent decision making of individuals in the society. -

Sarbari, PO - Neturia, Dist - Purulia, West Bengal - 723121 Website
PANCHAKOT MAHAVIDYALAYA Sarbari, PO - Neturia, Dist - Purulia, West Bengal - 723121 Website : www.panchakotmv.com SELF STUDY REPORT (Cycle 1) July, 2014 Submitted to: National Assessment and Accreditation Council P.O- Box No. 1075, Nagarbhavi, Bangalore-560 072, India. NAAC ‐ Accreditation Self Study Report 2014 Sl. No. Item Page No. 1 Forwarding Letter for uploading SSR 2 2 Abbreviations used 3 3 Committee for SSR preparation 4 4 Executive Summary & SWOC 5 5 Profile of the College 11 6 Criteria-wise Inputs 23 Criterion I: Curricular Aspects 24 Criterion II: Teaching-Learning and Evaluation 35 Criterion III: Research, Consultancy and Extension 69 Criterion IV: Infrastructure and Learning Resources 89 Criterion V: Student Support and Progression 104 Criterion VI: Governance, Leadership and Management 115 Criterion VII: Innovation and Best Practices 135 7 Evaluative Reports of the Departments 143 8 Declaration by the Head of the Institution 224 9 Annexure 225 1 PANCHAKOT MAHAVIDYALAYA, Sarbari, Neturia, Purulia, W.B. NAAC ‐ Accreditation Self Study Report 2014 2 PANCHAKOT MAHAVIDYALAYA, Sarbari, Neturia, Purulia, W.B. NAAC ‐ Accreditation Self Study Report 2014 ABBREVIATIONS (We Used) AIDS = Acquired Immuno-Deficiency Syndrome APC = Assembled Personal Computer BEUP = Bidhayak Elaka Unnayan Prakalpa BPC = Branded Personal Computer BSNL = Bharat Sanchar Nigam Limited CAS = Career Advancement Scheme DSLR = Digital Single Lens Reflex DST = Department of Science and Technology FC = Financially Challenged GB = Governing Body HED = Higher Education -

Village and Town Directory, Puruliya, Part XII-A , Series-26, West Bengal
CENSUS OF INDIA 1991 SERIES -26 WEST BENGAL DISTRICT CENSUS HANDBOOK PART XII-A VILLAGE AND TOWN DIRECTORY PURULIYA DISTRICT DIRECTORATE OF CENSUS OPERATIONS WEST BENGAL Price Rs. 30.00 PUBLISHED BY THE CONTROLLER GOVERNMENT PRINTING, WEST BENGAL AND PRINTED BY SARASWATY PRESS LTD. 11 B.T. ROAD, CALCUTTA -700056 CONTENTS Page No. 1. Foreword i-ii 2. Preface iii-iv 3. Acknowledgements v-vi 4. Important Statistics vii-viii 5. Analytical note and Analysis of Data ix-xxxiii Part A - Village and Town Directory 6. Section I - Village Directory Note explaining the Codes used in the Village Directory 3 (1) Hura C.D. Block 4-9 (a) Village Directory (2) Punch a C.D. Block 10-15 (a) Village Directory (3) Manbazar - I C.D. Block 16 - 29 (a) Village Directory (4) Manbazar -II C.D. Block 30- 41 (a) Village Directory (5) Raghunathpur - I C.D. Block 42-45 (a) Village Directory (6) Raghunathpur - II C.D. Block 46 - 51 (a) Village Directory (7) Bagmundi C.D. Block 52- 59 (a) Village Directory (a) Arsha C.D. Block 60-65 (a) Village Directory (9) Bundwan C.D. Block 66-73 (a) Village Directory (10) Jhalda -I C.D. Block 74 - 81 (a) Village Directory (11) Jhalda -II C.D. Block 82-89 (a) Village Directory (12) Neturia C.D. Block 90-95 (a) Village Directory (13) Kashipur C.O. Block 96 -107 (a) Village Directory (14) Santuri C.D. Block 108-115 (a) Village Directory (15) Para C.O. Block 116 -121 (a) Village Directory Page No. (16) Purulia -I C.D. -

Active Containment Zone As on 05-10-2020 District: Purulia
District: Purulia Active Containment Zone as on 05-10-2020 Sl. Block/ Operative Police Station Details of Containment Zone No. Municipality Upto 1 Baghmundi Baghmundi House of Budhu Paramanik, Tunturi Suisa 05.10.2020 2 Baghmundi Baghmundi House of Krishna Paramanik, Tunturi, Suisa 05.10.2020 3 Baghmundi Baghmundi House of Bibek Rouj, Tunturi, Suisa 05.10.2020 4 Baghmundi Baghmundi House of Samir Kuiry, Gagi, Suisa 05.10.2020 5 Manbazar-I Manbazar House of Joydeb Sing, Male, 32 Yrs, Village - Kadma, G.P. - Bamni Majhihira 05.10.2020 HOUSE OF ABANI MAHATO,VILL+PO-BHUTAM,PS-PUNCHA,DIST-PURULIA,PIN-723151,GP- 6 PUNCHA PUNCHA 05.10.2020 NAPARA 7 PARA PARA House of Anirban Sahababu,Village: Jabarrah under JJ-I GP 05.10.2020 8 Jhalda-I Block Jhalda House of Aktar Momin at Kenduadih village under Tulin GP 05.10.2020 9 Jhalda-I Block Jhalda House of Bijay Kumar at Chamardag village under Tulin GP 05.10.2020 10 Jhalda-I Block Jhalda House of Biswaketu Mahato at Patradih village of Pusti GP 05.10.2020 11 Jhalda-I Block Jhalda House of Ajay Darman at Jargo village under Illo Jargo GP 05.10.2020 House of Jadu Chandra, Covid positive- BALA CHANDRA,MAYA CHANDRA, SONALI 12 Jhalda Municipality Jhalda CHANDRA, RUPALI CHANDRA, SAMIR MAHATO, SUVIJIT CHANDRA, FELU CHANDRA, 05.10.2020 JHULAN CHANDRA, Jhalda Mandir Para 13 Jhalda Municipality Jhalda House of Gopal Paramanik Jhalda Mandir Para 05.10.2020 14 Jhalda Municipality Jhalda House of Krishnapada Das Jhalda Mandir Para 05.10.2020 15 Jhalda Municipality Jhalda House of Riya Keshri Jhalda Mandir Para 05.10.2020 16 Jhalda Municipality Jhalda House of Monotosh Roy Jhalda word no.-4 05.10.2020 17 Raghunathpur-II Raghunathpur House of Bela Bauri, Vill-Narira, Post-Nutandih, Dist.-Purulia 05.10.2020 18 Arsha Arsha House of Madhab Mahato S/o- Biswanath Mahato at Kishanpur under Mankiary G.P. -

Brief Summary of the Project
Brief Summary of the Project The proposed expansion of Ferro Alloys Plant, Iron Ore Beneficiation/Pellet Plant Integrated Steel Plant through secondary route of steel manufacture and Cement Grinding Plant will be located at village Nabagram, PO: Digha, Development Block: Neturia, Dist: Puruliya, West Bengal. Total land acquired for this project by M/s Ispat Damodar Pvt. Ltd. is 78 Acres in which a Sponge Iron Plant with 2 X 100 TPD kilns, SMS Plant with induction furnace of 2 X 4 T & 1 X 8 T capacity, a Ferroalloy plant of 4 X 7.5 MVA capacity and a Waste Heat Recovery based power plant of 8 MW capacity are already in operation. The existing plant has been operating since 2006. The company proposes to further acquire 25 acres of land for the expansion purpose. The acquisition will involve mostly private land. The site is accessible by Sarbari – Panchet Road which connects SH-5 (Purulia to Asansol via Dishergarh). Thus the site is having advantages of proximity to 2 Coalfields – i. e. Ranigunj Coal field of ECL on one side and Dhanbad coalfields of BCCL on the other. Nearest Railway station is Madhukunda on S.E. Railway which is about 10 Km. from the site. Nearest sea port is at Haldia at aerial distance of170 KM from the project site. Nearest Air Port is at Andal at a distance of 75 KM and Netaji Subhas Chandra Bose airport at Kolkota, at an aerial distance of 270KM from project site. Proposed expansion project cost is estimated for Rs.190.00Crores. -

Name of DDO/Hoo ADDRESS-1 ADDRESS CITY PIN SECTION REF
Name of DDO/HoO ADDRESS-1 ADDRESS CITY PIN SECTION REF. NO. BARCODE DATE THE SUPDT OF POLICE (ADMIN),SPL INTELLIGENCE COUNTER INSURGENCY FORCE ,W B,307,GARIA GROUP MAIN ROAD KOLKATA 700084 FUND IX/OUT/33 ew484941046in 12-11-2020 1 BENGAL GIRL'S BN- NCC 149 BLCK G NEW ALIPUR KOLKATA 0 0 KOLKATA 700053 FD XIV/D-325 ew460012316in 04-12-2020 2N BENAL. GIRLS BN. NCC 149, BLOCKG NEW ALIPORE KOL-53 0 NEW ALIPUR 700053 FD XIV/D-267 ew003044527in 27-11-2020 4 BENGAL TECH AIR SAQ NCC JADAVPUR LIMIVERSITY CAMPUS KOLKATA 0 0 KOLKATA 700032 FD XIV/D-313 ew460011823in 04-12-2020 4 BENGAL TECH.,AIR SQN.NCC JADAVPUR UNIVERSITY CAMPUS, KOLKATA 700036 FUND-VII/2019-20/OUT/468 EW460018693IN 26-11-2020 6 BENGAL BATTALION NCC DUTTAPARA ROAD 0 0 N.24 PGS 743235 FD XIV/D-249 ew020929090in 27-11-2020 A.C.J.M. KALYANI NADIA 0 NADIA 741235 FD XII/D-204 EW020931725IN 17-12-2020 A.O & D.D.O, DIR.OF MINES & MINERAL 4, CAMAC STREET,2ND FL., KOLKATA 700016 FUND-XIV/JAL/19-20/OUT/30 ew484927906in 14-10-2020 A.O & D.D.O, O/O THE DIST.CONTROLLER (F&S) KARNAJORA, RAIGANJ U/DINAJPUR 733130 FUDN-VII/19-20/OUT/649 EW020926425IN 23-12-2020 A.O & DDU. DIR.OF MINES & MINERALS, 4 CAMAC STREET,2ND FL., KOLKATA 700016 FUND-IV/2019-20/OUT/107 EW484937157IN 02-11-2020 STATISTICS, JT.ADMN.BULDS.,BLOCK-HC-7,SECTOR- A.O & E.O DY.SECY.,DEPTT.OF PLANNING & III, KOLKATA 700106 FUND-VII/2019-20/OUT/470 EW460018716IN 26-11-2020 A.O & EX-OFFICIO DY.SECY., P.W DEPTT. -

CONSOLIDATED DAILY ARREST REPORT DATED 12.04.2021 Father/ District/PC Name PS of District/PC SL
CONSOLIDATED DAILY ARREST REPORT DATED 12.04.2021 Father/ District/PC Name PS of District/PC SL. No Alias Sex Age Spouse Address Ps Name Name of Case/ GDE Ref. Accused residence of residence Name Accused Dakshin Parokata Kumargram PS Bishadu Lt. Charan Lalpool PS: Case No : 64/21 1 M 62 Samuktala Alipurduar Kumargram Alipurduar Barman Barman Samuktala US-46A Bengal Dist.: Excise Act, 1909 Alipurduar Aswini Nagar, Madarihat PS Ward 1 PS: Case No : 48/21 Pabitra Anil 2 M 30 Madarihat Madarihat Alipurduar Madarihat Alipurduar US- Sutradhar Sutradhar Dist.: 341/326/326/3 Alipurduar 07 IPC Madarihat PS Aswini Nagar, Case No : 47/21 Raj Kumar 3 Rajib M 30 Dipak Das Satipara PS: Baduria Madarihat Alipurduar US- Das Baduria 448/323/326/3 07 IPC Falakata PS Harinathpur Case No : Nirmal Lt Kachu PS: Falakata 122/21 US- 4 M 35 Falakata Alipurduar Falakata Alipurduar Das Das Dist.: 341/506/448/3 Alipurduar 23/326/307/35 4/34 IPC Falakata PS Khowchand Case No : Biplab Bholanath Para PS: 5 M 32 Falakata Alipurduar Falakata Alipurduar 124/21 US- Das Das Falakata Dist.: 341/325/379/5 Alipurduar 06/34 IPC Falakata PS Khowchand Lt Case No : Bholanath Para PS: 6 M 54 Manmoha Falakata Alipurduar Falakata Alipurduar 124/21 US- Das Falakata Dist.: n Das 341/325/379/5 Alipurduar 06/34 IPC Alipurduar Capatali PS: women PS Case Alipurduar Alipurduar 7 Sajal Das M 26 Satish Das Alipurduar Alipurduar Alipurduar No : 49/21 US- Dist.: women 448/323/354/5 Alipurduar 09/34 IPC TAPSIKHATA Sukumar PS: Alipurduar Alipurduar PS 8 Subal DEBNATH M 23 Alipurduar Alipurduar Alipurduar Alipurduar Debnath Dist.: GDE No. -
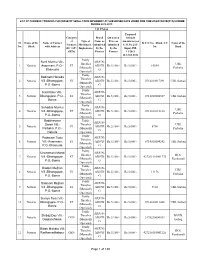
Ota Final List 1St Phase
LIST OF FARMERS TOWARDS PURCHASE OF SMALL FARM IMPLEMENT AT SUBSIDISED RATE UNDER ONE TIME ASSISTANCE(OTA)SCHEME DURING 2012-2013 1st Phase Proposed Category Brand Quotation Subsidy of Type of Name as Price as Amount as per Sl. Name of the Name of Farmer K.C.C.No. / Bank A/C Name of the Farmers Machinary / submitted submitted G.O.No.233- No. Block with Address No. Bank (SC / ST / Implements by the by the Input-9M- GEN) Farmer Farmer 11/2013 dt.12.02.2013 Paddy Sunil Murmu Vill.- ARJUN- Thresher UBI, 1 Neturia Asanmani, P.O.- ST AR07D Rs.5,100/- Rs.5,000/- 10054 (Manually Parbelia Bhamuria G Operated) Paddy Bodinath Hansda ARJUN- Thresher 2 Neturia Vill.-Dhangajore, ST AR07D Rs.5,100/- Rs.5,000/- 0714010017091 UBI, Sarbari (Manually P.O.-Bonra G Operated) Paddy Kati Kisku Vill.- ARJUN- Thresher 3 Neturia Dhangajore, P.O.- ST AR07D Rs.5,100/- Rs.5,000/- 0714010104517 UBI, Sarbari (Manually Bonra G Operated) Paddy Sahadeb Murmu ARJUN- Thresher UBI, 4 Neturia Vill.-Dhangajore, ST AR07D Rs.5,100/- Rs.5,000/- 0712010115100 (Manually Parbelia P.O.-Bonra G Operated) Buddheswar Paddy ARJUN- Soren Vill.- Thresher UBI, 5 Neturia ST AR07D Rs.5,100/- Rs.5,000/- 8708 Parbelia, P.O.- (Manually Parbelia G Neturia Operated) Paddy Patamani Tudu ARJUN- Thresher 6 Neturia Vill.-Asanmani, ST AR07D Rs.5,100/- Rs.5,000/- 0714010104242 UBI, Sarbari (Manually P.O.-Bhamuria G Operated) Paddy Chandmani Mandi ARJUN- Thresher BOI, 7 Neturia Vill.-Dhangajore, ST AR07D Rs.5,100/- Rs.5,000/- 427201110001776 (Manually Ramkanali P.O.-Bonra G Operated) Paddy Badani Mejhan -

Active Containment Zone As on 23-09-2020 District: Purulia
District: Purulia Active Containment Zone as on 23-09-2020 Sl. Block / Operative Police Station Details of Containment Zone No. Municipality Upto 1 Joypur Joypur House of Ganesh Swarnakar, Garh Joypur, Purulia 23.09.2020 2 Raghunathpur-I Raghunathpur House of Manik Das Dubradih, Sanka Ps- Adra Pin 723121 23.09.2020 House of Moumita Mukherjee c/o of Samir Mukherjee Raghunathpur Municipality ward no 13, 3 Raghunathpur-I Raghunathpur 23.09.2020 near Karmatirtha Dist-Purulia, PIN-723133 House of Lalita Bauri c/o Gurupada Bauri near Chinpina football ground Chinpina , Khajura 4 Raghunathpur-I Raghunathpur 23.09.2020 Raghunathpur 723133 House of Goutam Bauri(34), Father's Name-Ajoy Bauri(66),Vill+PO- Babugram under 5 Raghunathpur-I Raghunathpur 23.09.2020 Raghunathpur-I Dev. Block, PS- Raghunathpur, Dist-Purulia, PIN-723133 House of Aswani Bauri(20), Father's Name-Purna Chandra Bauri,Vill+PO- Krishnapur under 6 Raghunathpur-I Raghunathpur Babugram Gram Panchayat within Raghunathpur-I Dev. Block, PS- Raghunathpur, Dist-Purulia, 23.09.2020 PIN-723133 House of Mrinmoy Majee(23), Father's Name-Magaaram Majee(48),Vill+PO- Babugram(Chasa 7 Raghunathpur-I Raghunathpur 23.09.2020 Para) under Raghunathpur-I Dev. Block, PS- Raghunathpur, Dist-Purulia, PIN-723133 House of Payel Ghoshal c/o Aditya Ghoshal Raghunathpur municipality ward no-1 near kebal kali 8 Raghunathpur-I Raghunathpur 23.09.2020 mandir Raghunathpur PS, Pin-723133 House of Monima Rajak c/o - Late Haripada Rajak Rnpur Municipality ward no-1, near kebel kali 9 Raghunathpur-I Raghunathpur 23.09.2020 Mandir , Pin- 723133 10 Raghunathpur-II Raghunathpur House of Bidyut Mishra, Moutorh 23.09.2020 11 Kashipur Kashipur Sisir Mandal House, Loharpara Under Kashipur GP of Kashipur Development Block. -

ENVIRONMENTAL STATEMENT in FORM-V (Under Rule-14, Environmental (Protection) Rules, 1986)
ENVIRONMENTAL STATEMENT IN FORM-V (Under Rule-14, Environmental (Protection) Rules, 1986) (2018-2019) FOR CLUSTER NO. – 6 (GROUP OF MINES) Sodepur Area Eastern Coalfields Limited Prepared at Regional Institute – I Central Mine Planning & Design Institute Ltd. (A Subsidiary of Coal India Ltd.) G. T. Road (West End) Asansol - 713 304 CMPDI ISO 9001:2015 Company Environmental Statement (Form-V) Cluster No. – 6 (Group of Mines) 2018-19 ENVIRONMENTAL STATEMENT FORM – V Environmental statement for the financial year ending 31st March, 2019 Cluster No. – 6 FOR THE YEAR: 2018-19 CONTENTS SL.NO. CHAPTER PARTICULARS PAGE NO. 1 CHAPTER-I INTRODUCTION 2-6 2 CHAPTER-II ENVIRONMENTAL STATEMENT FORM-V (PART A TO I ) 7-17 LIST OF ANNEXURES ANNEXURE NO. PARTICULARS PAGE NO. I AMBIENT AIR QUALITY 18-24 II NOISE LEVEL REPORT 25 III WATER QUALITY REPORT – MINE WATER QUALITY 26-32 AND GROUND WATER QUALITY IV WELL WATER LEVEL REPORT 33 PLATES I LOCATION PLAN II PLAN SHOWING LOCATION OF MONITORING STATIONS 1 Environmental Statement (Form-V) Cluster No. – 6 (Group of Mines) 2018-19 CHAPTER – I INTRODUCTION 1.1 GENESIS: The Gazette Notification vide G.S.R No. 329 (E) dated 13th March, 1992 and subsequently renamed to ‘Environmental Statement’ vide Ministry of Environment & Forests (MOEF), Govt. of India gazette notification No. G.S.R No. 386 (E) Dtd. 22nd April’93 reads as follows. “Every person carrying on an industry, operation or process requiring consent under section 25 of the Water Act, 1974 or under section 21 of the Air Act, 1981 or both or authorisation under the Hazardous Waste Rules, 1989 issued under the Environmental Protection Act, 1986 shall submit an Environmental Audit Report for the year ending 31st March in Form V to the concerned State Pollution Control Board on or before the 30th day of September every year.” In compliance with the above and in fulfillment of condition laid out in the Environment Clearance granted by MoEF&CC, the work of Environmental Statement for Cluster No.