Phylogenetic Patterns of Sexual Size Dimorphism in Turtles and Their Implications for Rensch’S Rule
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Evolution of Parental Effects When Selection Acts on Fecundity Versus Viability
bioRxiv preprint doi: https://doi.org/10.1101/741835; this version posted August 21, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. The evolution of parental effects when selection acts on fecundity versus viability Bram Kuijper1;3 & Rufus A. Johnstone2;4 20th August 2019 Abstract Most predictions on the evolution of adaptive parental effects and phenotypic mem- ory exclusively focus on the role of the abiotic environment. How parental effects are affected by population demography and life history is less well understood. To overcome this, we use an analytical model to assess whether selection acting on fecundity versus viability affects the evolution of parental effects in a viscous population experiencing a spatiotemporally varying environment. We find that parental effects commonly evolve in regimes of viability selection, but are less likely to evolve in regimes of fecundity se- lection. In regimes of viability selection, an individual’s phenotype becomes correlated with its local environment during its lifetime, as those individuals with a locally adapted phenotype are more likely to survive until parenthood. Hence, a parental phenotype rapidly becomes an informative cue about its local environment, favoring the evolution of parental effects. By contrast, in regimes of fecundity selection, locally maladapted and adapted parents survive at equal rates, so that the parental phenotype, by itself, is not informative about the local environment. Correlations between phenotype and envi- ronment still arise, but only when more fecund, locally adapted individuals leave more successfully established offspring to the local patch. -

A Review of Southern Iraq Herpetofauna
Vol. 3 (1): 61-71, 2019 A Review of Southern Iraq Herpetofauna Nadir A. Salman Mazaya University College, Dhi Qar, Iraq *Corresponding author: [email protected] Abstract: The present review discussed the species diversity of herpetofauna in southern Iraq due to their scientific and national interests. The review includes a historical record for the herpetofaunal studies in Iraq since the earlier investigations of the 1920s and 1950s along with the more recent taxonomic trials in the following years. It appeared that, little is known about Iraqi herpetofauna, and no comprehensive checklist has been done for these species. So far, 96 species of reptiles and amphibians have been recorded from Iraq, but only a relatively small proportion of them occur in the southern marshes. The marshes act as key habitat for globally endangered species and as a potential for as yet unexplored amphibian and reptile diversity. Despite the lack of precise localities, the tree frog Hyla savignyi, the marsh frog Pelophylax ridibunda and the green toad Bufo viridis are found in the marshes. Common reptiles in the marshes include the Caspian terrapin (Clemmys caspia), the soft-shell turtle (Trionyx euphraticus), the Euphrates softshell turtle (Rafetus euphraticus), geckos of the genus Hemidactylus, two species of skinks (Trachylepis aurata and Mabuya vittata) and a variety of snakes of the genus Coluber, the spotted sand boa (Eryx jaculus), tessellated water snake (Natrix tessellata) and Gray's desert racer (Coluber ventromaculatus). More recently, a new record for the keeled gecko, Cyrtopodion scabrum and the saw-scaled viper (Echis carinatus sochureki) was reported. The IUCN Red List includes six terrestrial and six aquatic amphibian species. -

Temperature As a Modulator of Sexual Selection
Biol. Rev. (2020), pp. 000–000. 1 doi: 10.1111/brv.12632 Temperature as a modulator of sexual selection Roberto García-Roa1, Francisco Garcia-Gonzalez2,3, Daniel W.A. Noble4,5 and Pau Carazo1* 1Behaviour and Evolution, Ethology Lab, Cavanilles Institute of Biodiversity and Evolutionary Biology, University of Valencia, C/Catedrático José Beltrán 2, Paterna, Valencia, 46980, Spain 2Doñana Biological Station, Spanish Research Council CSIC, c/Americo Vespucio, 26, Isla de la Cartuja, Sevilla, 41092, Spain 3Centre for Evolutionary Biology, School of Biological Sciences, The University of Western Australia, 35 Stirling Highway, Crawley, Western Australia, 6009, Australia 4Ecology and Evolution Research Centre, School of Biological, Earth and Environmental Sciences, University of New South Wales, Sydney, New South Wales, 2052, Australia 5Division of Ecology and Evolution, Research School of Biology, The Australian National University, Canberra, Australian Capital Territory, 2061, Australia ABSTRACT A central question in ecology and evolution is to understand why sexual selection varies so much in strength across taxa; it has long been known that ecological factors are crucial to this. Temperature is a particularly salient abiotic ecological factor that modulates a wide range of physiological, morphological and behavioural traits, impacting individuals and populations at a global taxonomic scale. Furthermore, temperature exhibits substantial temporal variation (e.g. daily, seasonally and inter-seasonally), and hence for most species in the wild sexual selection will regularly unfold in a dynamic thermal environment. Unfortunately, studies have so far almost completely neglected the role of temperature as a mod- ulator of sexual selection. Here, we outline the main pathways through which temperature can affect the intensity and form (i.e. -

Green Sea Turtle in the New England Aquarium Has Been in Captivity Since 1970, and Is Believed to Be Around 80 Years Old (NEAQ 2013)
Species Status Assessment Class: Reptilia Family: Cheloniidae Scientific Name: Chelonia mydas Common Name: Green turtle Species synopsis: The green turtle is a marine turtle that was originally described by Linnaeus in 1758 as Testudo mydas. In 1868 Marie Firmin Bocourt named a new species of sea turtle Chelonia agassizii. It was later determined that these represented the same species, and the name became Chelonia mydas. In New York, the green turtle can be found from July – November, with individuals occasionally found cold-stunned in the winter months (Berry et al. 1997, Morreale and Standora 1998). Green turtles are sighted most frequently in association with sea grass beds off the eastern side of Long Island. They are observed with some regularity in the Peconic Estuary (Morreale and Standora 1998). Green turtles experienced a drastic decline throughout their range during the 19th and 20th centuries as a result of human exploitation and anthropogenic habitat degradation (NMFS and USFWS 1991). In recent years, some populations, including the Florida nesting population, have been experiencing some signs of increase (NMFS and USFWS 2007). Trends have not been analyzed in New York; a mark-recapture study performed in the state from 1987 – 1992 found that there seemed to be more green turtles at the end of the study period (Berry et al. 1997). However, changes in temperature have lead to an increase in the number of cold stunned green turtles in recent years (NMFS, Riverhead Foundation). Also, this year a record number of nests were observed at nesting beaches in Flordia (Mote Marine Laboratory 2013). 1 I. -
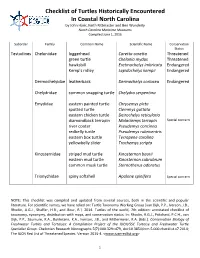
N.C. Turtles Checklist
Checklist of Turtles Historically Encountered In Coastal North Carolina by John Hairr, Keith Rittmaster and Ben Wunderly North Carolina Maritime Museums Compiled June 1, 2016 Suborder Family Common Name Scientific Name Conservation Status Testudines Cheloniidae loggerhead Caretta caretta Threatened green turtle Chelonia mydas Threatened hawksbill Eretmochelys imbricata Endangered Kemp’s ridley Lepidochelys kempii Endangered Dermochelyidae leatherback Dermochelys coriacea Endangered Chelydridae common snapping turtle Chelydra serpentina Emydidae eastern painted turtle Chrysemys picta spotted turtle Clemmys guttata eastern chicken turtle Deirochelys reticularia diamondback terrapin Malaclemys terrapin Special concern river cooter Pseudemys concinna redbelly turtle Pseudemys rubriventris eastern box turtle Terrapene carolina yellowbelly slider Trachemys scripta Kinosternidae striped mud turtle Kinosternon baurii eastern mud turtle Kinosternon subrubrum common musk turtle Sternotherus odoratus Trionychidae spiny softshell Apalone spinifera Special concern NOTE: This checklist was compiled and updated from several sources, both in the scientific and popular literature. For scientific names, we have relied on: Turtle Taxonomy Working Group [van Dijk, P.P., Iverson, J.B., Rhodin, A.G.J., Shaffer, H.B., and Bour, R.]. 2014. Turtles of the world, 7th edition: annotated checklist of taxonomy, synonymy, distribution with maps, and conservation status. In: Rhodin, A.G.J., Pritchard, P.C.H., van Dijk, P.P., Saumure, R.A., Buhlmann, K.A., Iverson, J.B., and Mittermeier, R.A. (Eds.). Conservation Biology of Freshwater Turtles and Tortoises: A Compilation Project of the IUCN/SSC Tortoise and Freshwater Turtle Specialist Group. Chelonian Research Monographs 5(7):000.329–479, doi:10.3854/crm.5.000.checklist.v7.2014; The IUCN Red List of Threatened Species. -

The Common Snapping Turtle, Chelydra Serpentina
The Common Snapping Turtle, Chelydra serpentina Rylen Nakama FISH 423: Olden 12/5/14 Figure 1. The Common Snapping Turtle, one of the most widespread reptiles in North America. Photo taken in Quebec, Canada. Image from https://www.flickr.com/photos/yorthopia/7626614760/. Classification Order: Testudines Family: Chelydridae Genus: Chelydra Species: serpentina (Linnaeus, 1758) Previous research on Chelydra serpentina (Phillips et al., 1996) acknowledged four subspecies, C. s. serpentina (Northern U.S. and Figure 2. Side profile of Chelydra serpentina. Note Canada), C. s. osceola (Southeastern U.S.), C. s. the serrated posterior end of the carapace and the rossignonii (Central America), and C. s. tail’s raised central ridge. Photo from http://pelotes.jea.com/AnimalFact/Reptile/snapturt.ht acutirostris (South America). Recent IUCN m. reclassification of chelonians based on genetic analyses (Rhodin et al., 2010) elevated C. s. rossignonii and C. s. acutirostris to species level and established C. s. osceola as a synonym for C. s. serpentina, thus eliminating subspecies within C. serpentina. Antiquated distinctions between the two formerly recognized North American subspecies were based on negligible morphometric variations between the two populations. Interbreeding in the overlapping range of the two populations was well documented, further discrediting the validity of the subspecies distinction (Feuer, 1971; Aresco and Gunzburger, 2007). Therefore, any emphasis of subspecies differentiation in the ensuing literature should be disregarded. Figure 3. Front-view of a captured Chelydra Continued usage of invalid subspecies names is serpentina. Different skin textures and the distinctive pink mouth are visible from this angle. Photo from still prevalent in the exotic pet trade for C. -

Turtles, All Marine Turtles, Have Been Documented Within the State’S Borders
Turtle Only four species of turtles, all marine turtles, have been documented within the state’s borders. Terrestrial and freshwater aquatic species of turtles do not occur in Alaska. Marine turtles are occasional visitors to Alaska’s Gulf Coast waters and are considered a natural part of the state’s marine ecosystem. Between 1960 and 2007 there were 19 reports of leatherback sea turtles (Dermochelys coriacea), the world’s largest turtle. There have been 15 reports of Green sea turtles (Chelonia mydas). The other two are extremely rare, there have been three reports of Olive ridley sea turtles (Lepidochelys olivacea) and two reports of loggerhead sea turtles (Caretta caretta). Currently, all four species are listed as threatened or endangered under the U.S. Endangered Species Act. Prior to 1993, Alaska marine turtle sightings were mostly of live leatherback sea turtles; since then most observations have been of green sea turtle carcasses. At present, it is not possible to determine if this change is related to changes in oceanographic conditions, perhaps as the result of global warming, or to changes in the overall population size and distribution of these species. General description: Marine turtles are large, tropical/subtropical, thoroughly aquatic reptiles whose forelimbs or flippers are specially modified for swimming and are considerably larger than their hind limbs. Movements on land are awkward. Except for occasional basking by both sexes and egg-laying by females, turtles rarely come ashore. Turtles are among the longest-lived vertebrates. Although their age is often exaggerated, they probably live 50 to 100 years. Of the five recognized species of marine turtles, four (including the green sea turtle) belong to the family Cheloniidae. -

Fecundity Selection Theory: Concepts and Evidence
bioRxiv preprint doi: https://doi.org/10.1101/015586; this version posted February 23, 2015. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Fecundity selection theory: concepts and evidence Daniel Pincheira-Donoso1,3 & John Hunt2 1Laboratory of Evolutionary Ecology of Adaptations, School of Life Sciences, University of Lincoln, Brayford Campus, Lincoln, LN6 7DL, Lincolnshire, United Kingdom 2Centre for Ecology and Conservation, College of Life and Environmental Sciences, University of Exeter, Cornwall Campus, Penryn, Cornwall, TR10 9EZ, UK 3Corresponding author: [email protected] ABSTRACT Fitness results from the optimal balance between survival, mating success and fecundity. The interactions between these three components of fitness vary importantly depending on the selective context, from positive covariation between them, to antagonistic pleiotropic relationships when fitness increases in one reduce fitness of others. Therefore, elucidating the routes through which selection shapes life history and phenotypic adaptations via these fitness components is of primary significance to understand ecological and evolutionary dynamics. However, while the fitness components mediated by natural (survival) and sexual (mating success) selection have extensively been debated from most possible perspectives, fecundity selection remains considerably -

71St Annual Meeting Society of Vertebrate Paleontology Paris Las Vegas Las Vegas, Nevada, USA November 2 – 5, 2011 SESSION CONCURRENT SESSION CONCURRENT
ISSN 1937-2809 online Journal of Supplement to the November 2011 Vertebrate Paleontology Vertebrate Society of Vertebrate Paleontology Society of Vertebrate 71st Annual Meeting Paleontology Society of Vertebrate Las Vegas Paris Nevada, USA Las Vegas, November 2 – 5, 2011 Program and Abstracts Society of Vertebrate Paleontology 71st Annual Meeting Program and Abstracts COMMITTEE MEETING ROOM POSTER SESSION/ CONCURRENT CONCURRENT SESSION EXHIBITS SESSION COMMITTEE MEETING ROOMS AUCTION EVENT REGISTRATION, CONCURRENT MERCHANDISE SESSION LOUNGE, EDUCATION & OUTREACH SPEAKER READY COMMITTEE MEETING POSTER SESSION ROOM ROOM SOCIETY OF VERTEBRATE PALEONTOLOGY ABSTRACTS OF PAPERS SEVENTY-FIRST ANNUAL MEETING PARIS LAS VEGAS HOTEL LAS VEGAS, NV, USA NOVEMBER 2–5, 2011 HOST COMMITTEE Stephen Rowland, Co-Chair; Aubrey Bonde, Co-Chair; Joshua Bonde; David Elliott; Lee Hall; Jerry Harris; Andrew Milner; Eric Roberts EXECUTIVE COMMITTEE Philip Currie, President; Blaire Van Valkenburgh, Past President; Catherine Forster, Vice President; Christopher Bell, Secretary; Ted Vlamis, Treasurer; Julia Clarke, Member at Large; Kristina Curry Rogers, Member at Large; Lars Werdelin, Member at Large SYMPOSIUM CONVENORS Roger B.J. Benson, Richard J. Butler, Nadia B. Fröbisch, Hans C.E. Larsson, Mark A. Loewen, Philip D. Mannion, Jim I. Mead, Eric M. Roberts, Scott D. Sampson, Eric D. Scott, Kathleen Springer PROGRAM COMMITTEE Jonathan Bloch, Co-Chair; Anjali Goswami, Co-Chair; Jason Anderson; Paul Barrett; Brian Beatty; Kerin Claeson; Kristina Curry Rogers; Ted Daeschler; David Evans; David Fox; Nadia B. Fröbisch; Christian Kammerer; Johannes Müller; Emily Rayfield; William Sanders; Bruce Shockey; Mary Silcox; Michelle Stocker; Rebecca Terry November 2011—PROGRAM AND ABSTRACTS 1 Members and Friends of the Society of Vertebrate Paleontology, The Host Committee cordially welcomes you to the 71st Annual Meeting of the Society of Vertebrate Paleontology in Las Vegas. -

Universidad Nacional Del Comahue Centro Regional Universitario Bariloche
Universidad Nacional del Comahue Centro Regional Universitario Bariloche Título de la Tesis Microanatomía y osteohistología del caparazón de los Testudinata del Mesozoico y Cenozoico de Argentina: Aspectos sistemáticos y paleoecológicos implicados Trabajo de Tesis para optar al Título de Doctor en Biología Tesista: Lic. en Ciencias Biológicas Juan Marcos Jannello Director: Dr. Ignacio A. Cerda Co-director: Dr. Marcelo S. de la Fuente 2018 Tesis Doctoral UNCo J. Marcos Jannello 2018 Resumen Las inusuales estructuras óseas observadas entre los vertebrados, como el cuello largo de la jirafa o el cráneo en forma de T del tiburón martillo, han interesado a los científicos desde hace mucho tiempo. Uno de estos casos es el clado Testudinata el cual representa uno de los grupos más fascinantes y enigmáticos conocidos entre de los amniotas. Su inconfundible plan corporal, que ha persistido desde el Triásico tardío hasta la actualidad, se caracteriza por la presencia del caparazón, el cual encierra a las cinturas, tanto pectoral como pélvica, dentro de la caja torácica desarrollada. Esta estructura les ha permitido a las tortugas adaptarse con éxito a diversos ambientes (por ejemplo, terrestres, acuáticos continentales, marinos costeros e incluso marinos pelágicos). Su capacidad para habitar diferentes nichos ecológicos, su importante diversidad taxonómica y su plan corporal particular hacen de los Testudinata un modelo de estudio muy atrayente dentro de los vertebrados. Una disciplina que ha demostrado ser una herramienta muy importante para abordar varios temas relacionados al caparazón de las tortugas, es la paleohistología. Esta disciplina se ha involucrado en temas diversos tales como el origen del caparazón, el origen del desarrollo y mantenimiento de la ornamentación, la paleoecología y la sistemática. -

Investigating Sexual Dimorphism in Ceratopsid Horncores
University of Calgary PRISM: University of Calgary's Digital Repository Graduate Studies The Vault: Electronic Theses and Dissertations 2013-01-25 Investigating Sexual Dimorphism in Ceratopsid Horncores Borkovic, Benjamin Borkovic, B. (2013). Investigating Sexual Dimorphism in Ceratopsid Horncores (Unpublished master's thesis). University of Calgary, Calgary, AB. doi:10.11575/PRISM/26635 http://hdl.handle.net/11023/498 master thesis University of Calgary graduate students retain copyright ownership and moral rights for their thesis. You may use this material in any way that is permitted by the Copyright Act or through licensing that has been assigned to the document. For uses that are not allowable under copyright legislation or licensing, you are required to seek permission. Downloaded from PRISM: https://prism.ucalgary.ca UNIVERSITY OF CALGARY Investigating Sexual Dimorphism in Ceratopsid Horncores by Benjamin Borkovic A THESIS SUBMITTED TO THE FACULTY OF GRADUATE STUDIES IN PARTIAL FULFILMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF SCIENCE DEPARTMENT OF BIOLOGICAL SCIENCES CALGARY, ALBERTA JANUARY, 2013 © Benjamin Borkovic 2013 Abstract Evidence for sexual dimorphism was investigated in the horncores of two ceratopsid dinosaurs, Triceratops and Centrosaurus apertus. A review of studies of sexual dimorphism in the vertebrate fossil record revealed methods that were selected for use in ceratopsids. Mountain goats, bison, and pronghorn were selected as exemplar taxa for a proof of principle study that tested the selected methods, and informed and guided the investigation of sexual dimorphism in dinosaurs. Skulls of these exemplar taxa were measured in museum collections, and methods of analysing morphological variation were tested for their ability to demonstrate sexual dimorphism in their horns and horncores. -

Natural Selection on Fecundity Variance in Subdivided Populations: Kin Selection Meets Bet Hedging
Copyright Ó 2007 by the Genetics Society of America DOI: 10.1534/genetics.106.066910 Natural Selection on Fecundity Variance in Subdivided Populations: Kin Selection Meets Bet Hedging Laurent Lehmann1 and Francxois Balloux Department of Genetics, University of Cambridge, CB2 3EH Cambridge, United Kingdom Manuscript received October 16, 2006 Accepted for publication February 5, 2007 ABSTRACT In a series of seminal articles in 1974, 1975, and 1977, J. H. Gillespie challenged the notion that the ‘‘fittest’’ individuals are those that produce on average the highest number of offspring. He showed that in small populations, the variance in fecundity can determine fitness as much as mean fecundity. One likely reason why Gillespie’s concept of within-generation bet hedging has been largely ignored is the general consensus that natural populations are of large size. As a consequence, essentially no work has investigated the role of the fecundity variance on the evolutionary stable state of life-history strategies. While typically large, natural populations also tend to be subdivided in local demes connected by migration. Here, we integrate Gillespie’s measure of selection for within-generation bet hedging into the inclusive fitness and game theoretic measure of selection for structured populations. The resulting framework demonstrates that selection against high variance in offspring number is a potent force in large, but structured populations. More generally, the results highlight that variance in offspring number will directly affect various life-history strategies, especially those involving kin interaction. The selective pressures on three key traits are directly investigated here, namely within-generation bet hedging, helping behaviors, and the evolutionary stable dispersal rate.