(Urva Auropunctata) by M. A
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Reproductive Traits of Two Sympatric Viviparous Skinks (Mabuya Macrorhyncha and Mabuya Ag/Lis) in a Brazilian Restinga Habitat
HERPETOLOGICAL JOURNAL, Vol. 9, pp. 43-53 (1999) REPRODUCTIVE TRAITS OF TWO SYMPATRIC VIVIPAROUS SKINKS (MABUYA MACRORHYNCHA AND MABUYA AG/LIS) IN A BRAZILIAN RESTINGA HABITAT CARLOS FREDERICO DUARTE ROCHA AND DAVOR VRCIBRADIC Setor de Ecologia, Instituto de Biologia, Universidade do Estado do Rio de Janeiro, Brazil The reproductive cycles, fat body cycles and some life-history traits of the sympatric viviparous skinks Mabuya macrorhyncha and M. agilis were compared in a seasonal "restinga" habitat of south-eastern Brazil. Both male and female reproductive and fatbody cycles are very similar between species, with gestation lasting 9-12 months and parturition occurring during the early wet season. Clutch size of M. macrorhyncha was smaller than that of M. ag ilis. Females mature at a larger size in M. macrorhyncha than in M. agilis, but males of both species appear to mature at similar sizes. In both species, females are larger than males, but the latter have proportionately larger heads. Reproductive traits ofM. ag ilis are typical ofNeotropical Mabuya, but those of M. macrorhyncha have some peculiarities, one of which (small clutch size) is believed to result from constraints imposed by its morphological adaptation (i.e. relatively flattened body plan) to bromelicolous habits. Key words: Reproduction, life history, Mabuya, Brazilian skink INTRODUCTION which present differences may highlight those histori The genus Mabuya is one of the most speciose and cal forces that may select for certain reproductive widely distributed genera of the Scincidae, with about characteristics. Indeed, differences in reproductive 90 species found over most of tropical Africa,Asia , and characteristics among sympatric and allopatric populations of congeners have been reported for this America (Shine, 1985; Nussbaum & Rax worthy, 1994). -

(2007) a Photographic Field Guide to the Reptiles and Amphibians Of
A Photographic Field Guide to the Reptiles and Amphibians of Dominica, West Indies Kristen Alexander Texas A&M University Dominica Study Abroad 2007 Dr. James Woolley Dr. Robert Wharton Abstract: A photographic reference is provided to the 21 reptiles and 4 amphibians reported from the island of Dominica. Descriptions and distribution data are provided for each species observed during this study. For those species that were not captured, a brief description compiled from various sources is included. Introduction: The island of Dominica is located in the Lesser Antilles and is one of the largest Eastern Caribbean islands at 45 km long and 16 km at its widest point (Malhotra and Thorpe, 1999). It is very mountainous which results in extremely varied distribution of habitats on the island ranging from elfin forest in the highest elevations, to rainforest in the mountains, to dry forest near the coast. The greatest density of reptiles is known to occur in these dry coastal areas (Evans and James, 1997). Dominica is home to 4 amphibian species and 21 (previously 20) reptile species. Five of these are endemic to the Lesser Antilles and 4 are endemic to the island of Dominica itself (Evans and James, 1997). The addition of Anolis cristatellus to species lists of Dominica has made many guides and species lists outdated. Evans and James (1997) provides a brief description of many of the species and their habitats, but this booklet is inadequate for easy, accurate identification. Previous student projects have documented the reptiles and amphibians of Dominica (Quick, 2001), but there is no good source for students to refer to for identification of these species. -

Assessment of Species Richness and Relative Abundance of Small Carnivores in Natural Forest and Shrub Thickets at the University of Dodoma
The University of Dodoma University of Dodoma Institutional Repository http://repository.udom.ac.tz Natural Sciences Master Dissertations 2013 Assessment of species richness and relative abundance of small carnivores in natural forest and shrub thickets at the University of Dodoma Mwiyoha, Baraka D. The University of Dodoma Mwiyoha, B. D. (2013). Assessment of species richness and relative abundance of small carnivores in natural forest and shrub thickets at the University of Dodoma. Dodoma: The University of Dodoma http://hdl.handle.net/20.500.12661/1518 Downloaded from UDOM Institutional Repository at The University of Dodoma, an open access institutional repository. ASSESSMENT OF SPECIES RICHNESS AND RELATIVE ABUNDANCE OF SMALL CARNIVORES IN NATURAL FOREST AND SHRUB THICKETS AT THE UNIVERSITY OF DODOMA By Baraka David Mwiyoha Dissertation Submitted in Partial Fulfilment of the Requirements for the Degree of Masters of Science in Biodiversity Conservation of the University of Dodoma. The University of Dodoma October, 2013 CERTIFICATION The undersigned certify that she has read and hereby recommend for acceptance by the University of Dodoma dissertation entitled Assessment of species richness and relative abundance of small carnivores in natural forest and shrub thickets at the University of Dodoma in fulfillment of the requirements for the degree of masters of science in biodiversity conservation of the University of Dodoma. …………………………………… Dr. Shyamala Ratnayeke (SUPERVISOR) Date………………………………… i DECLARATION AND COPYRIGHT I, Baraka David Mwiyoha, declare that this dissertation is my own original work and that it has not been presented and will not be presented to any other university for a similar or any other degree award. -

Hand-Rearing and Rehabilitation of Orphaned Palm Squirrels, Funambulus Sp
Hand-rearing and Rehabilitation of Orphaned Palm Squirrels, Funambulus sp. Devna Arora The Palm Squirrel A young 5-striped Indian palm squirrel Palm squirrels are palm-sized rodents with thick, bushy tails that belong to the genus Funambulus and the subfamily Calloscuirinae, a subfamily of squirrels found in Asia. The genus Funambulus comprises of 5 species of squirrels widely distributed in the Indian subcontinent with the subgenus of Prasadsciurus found right up to Iran. For ease of classification and understanding of behaviour, I prefer to group these squirrels based on their proximity to human settlements. The two species of palm squirrels commonly found in urban, suburban and rural landscapes are: • Indian 3-Striped Palm Squirrel (Funambulus palmarum) which has a more southern distribution and is commonly found in peninsular India and Sri Lanka. Rehabber’s Den © 2013 1 Hand-rearing and rehabilitation of orphaned palm squirrels, Funambulus sp. • Indian 5-Striped Palm Squirrel (Funambulus pennantii) which has a more northern distribution in India and in commonly found in central and northern India as well as Nepal, Pakistan and Iran. The two species can easily be distinguished by counting the number of paler coloured stripes on the squirrel’s back. The squirrels range from 22.5 cm to 40 cm in length, which includes a tail of 11–12 cm long, and weigh between 100–200 gm. Data suggests that palm squirrels live on average for 5-6 years in captivity although individuals have been known to live longer. They may only breed seasonally in the northern distribution of their ranges but breed all-year round otherwise. -

Morphological Aspects of the Brain in the Indian Grey Mongoose (Herpestes Edwardsii)
Iranian Journal of Veterinary Received: 2020- Mar- 06 Accepted after revision: 2020- Nov- 01 Science and Technology Published online: 2021- Feb- 27 Short communication DOI: 10.22067/ijvst.2020.39237 Morphological aspects of the brain in the Indian grey mongoose (Herpestes Edwardsii) Babak Rasouli, Soghra Gholami, Younes Kamali Department of Basic Sciences, School of Veterinary Medicine, Shiraz University, Shiraz, Iran. ABSTRACT Mongoose is a common name for 29 to 34 species in 14 genera of the family Herpestidae which are found in vast areas of southwestern Asia, especially southern Iran. Anatomical and morphological studies of the brain have always been of interest to the researchers in the field of anatomy, due to its high importance in various fields of veterinary and zoology. Because of the lack of information about the brain structure in wild carnivores, the present study was conducted to better understand the morphological features in Indian grey mongoose. For this purpose, 4 carcasses of adult mongooses were used. They were found in different areas of Fars province. The mongooses had died due to natural causes. The brain was carefully separated from the skull and the measurements and observations were made on different parts of it. In this study, it wa found that the brain's structure has an ovoid appearance. Also, distinguished olfactory bulbs, deep transverse and longitudinal fissures, and relatively large cerebellar vermis were observed. Accord- ing to the current study, it can be concluded that the anatomical features of the brain in the mongoose are similar to those of other carnivores and are in perfect harmony with the sensory and motor capabilities of the animal. -

1. Biological Environment 1.1
EB Report for Expansion of Corporate Office Building, Noida (U.P.) ……….………………………………………………………………………………………………………………………………………………………………………………… 1. Biological Environment 1.1. Introduction Biodiversity reflects the potential of a regional ecosystem. Biota of a particular area is considered as indicators of the environment as they quickly respond not only to one environmental factor but also an interactive group of factors. These communities influence and react sensitively to changes in the balance of environmental stresses. Conservation of the biodiversity is essential for the sustainable development. Before starting any Environmental Impact Assessment study, it is necessary to identify the baseline of relevant environmental parameters which are likely to be affected as a result of the operation of the proposed project. A similar approach has been adopted for conducting the study on biological environment for this project. Both terrestrial and aquatic ecosystems have been studied to understand the biological environment nearby the project site. The study was conducted in the project area to assess all possible consequences on the biological environment. The present study is highlighting the various issues pertaining to floristic diversity and the faunal wealth in the core area i.e. Expansion of Corporate Office Building at Sector-16A, Film City, Noida (U.P.) and buffer zone i.e. area within 10 km radius. 1.1.1. Description of Study Area The present project proposes modification of the Expansion of Corporate Office Building which is located Sector-16A, Film City, Noida (U.P.) under the Seismic Zone –IV as per IS 1893 (Part I): 2002 (indicating high damage risk zone). The buildings will be designed as earthquake resistant and comply with IS specifications. -

Small Carnivores in Tinjure-Milke-Jaljale, Eastern Nepal
SMALL CARNIVORES IN TINJURE-MILKE-JALJALE, EASTERN NEPAL The content of this booklet can be used freely with permission for any conservation and education purpose. However we would be extremely happy to get a hard copy or soft copy of the document you have used it for. For further information: Friends of Nature Kathmandu, Nepal P.O. Box: 23491 Email: [email protected], Website: www.fonnepal.org Facebook: www.facebook.com/fonnepal2005 First Published: April, 2018 Photographs: Friends of Nature (FON), Jeevan Rai, Zaharil Dzulkafly, www.pixabay/ werner22brigitte Design: Roshan Bhandari Financial support: Rufford Small Grants, UK Authors: Jeevan Rai, Kaushal Yadav, Yadav Ghimirey, Som GC, Raju Acharya, Kamal Thapa, Laxman Prasad Poudyal and Nitesh Singh ISBN: 978-9937-0-4059-4 Acknowledgements: We are grateful to Zaharil Dzulkafly for his photographs of Marbled Cat, and Andrew Hamilton and Wildscreen for helping us get them. We are grateful to www.pixabay/werner22brigitte for giving us Binturong’s photograph. We thank Bidhan Adhikary, Thomas Robertson, and Humayra Mahmud for reviewing and providing their valuable suggestions. Preferred Citation: Rai, J., Yadav, K., Ghimirey, Y., GC, S., Acharya, R., Thapa, K., Poudyal, L.P., and Singh, N. 2018. Small Carnivores in Tinjure-Milke-Jaljale, Eastern Nepal. Friends of Nature, Nepal and Rufford Small Grants, UK. Small Carnivores in Tinjure-Milke-Jaljale, Eastern Nepal Why Protect Small Carnivores! Small carnivores are an integral part of our ecosystem. Except for a few charismatic species such as Red Panda, a general lack of research and conservation has created an information gap about them. I am optimistic that this booklet will, in a small way, be the starting journey of filling these gaps in our knowledge bank of small carnivore in Nepal. -

Malawi Trip Report 12Th to 28Th September 2014
Malawi Trip Report 12th to 28th September 2014 Bohm’s Bee-eater by Keith Valentine Trip Report compiled by Tour Leader: Keith Valentine RBT Malawi Trip Report September 2014 2 Top 10 Birds: 1. Scarlet-tufted Sunbird 2. Pel’s Fishing Owl 3. Lesser Seedcracker 4. Thyolo Alethe 5. White-winged Apalis 6. Racket-tailed Roller 7. Blue Swallow 8. Bohm’s Flycatcher 9. Babbling Starling 10. Bohm’s Bee-eater/Yellow-throated Apalis Top 5 Mammals: 1. African Civet 2. Four-toed Elephant Shrew 3. Sable Antelope 4. Bush Pig 5. Side-striped Jackal/Greater Galago/Roan Antelope/Blotched Genet Trip Summary This was our first ever fully comprehensive tour to Malawi and was quite simply a fantastic experience in all respects. For starters, many of the accommodations are of excellent quality and are also situated in prime birding locations with a large number of the area’s major birding targets found in close proximity. The food is generally very good and the stores and lodges are for the most part stocked with decent beer and a fair selection of South African wine. However, it is the habitat diversity that is largely what makes Malawi so good from a birding point of view. Even though it is a small country, this good variety of habitat, and infrastructure that allows access to these key zones, insures that the list of specials is long and attractive. Our tour was extremely successful in locating the vast majority of the region’s most wanted birds and highlights included Red-winged Francolin, White-backed Night Heron, African Cuckoo-Hawk, Western Banded Snake -

Ecology of the Small Indian Mongoose (Herpestes Auropunctatus) in North America
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln USDA National Wildlife Research Center - Staff U.S. Department of Agriculture: Animal and Publications Plant Health Inspection Service 2018 Ecology of the Small Indian Mongoose (Herpestes auropunctatus) in North America Are R. Berentsen USDA National Wildlife Research Center, [email protected] William C. Pitt Smithsonian Institute Robert T. Sugihara USDA/APHIS/WS/National Wildlife Research Center Follow this and additional works at: https://digitalcommons.unl.edu/icwdm_usdanwrc Part of the Life Sciences Commons Berentsen, Are R.; Pitt, William C.; and Sugihara, Robert T., "Ecology of the Small Indian Mongoose (Herpestes auropunctatus) in North America" (2018). USDA National Wildlife Research Center - Staff Publications. 2034. https://digitalcommons.unl.edu/icwdm_usdanwrc/2034 This Article is brought to you for free and open access by the U.S. Department of Agriculture: Animal and Plant Health Inspection Service at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in USDA National Wildlife Research Center - Staff Publications by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. U.S. Department of Agriculture U.S. Government Publication Animal and Plant Health Inspection Service Wildlife Services Ecology of the Small 12 Indian Mongoose (Herpestes auropunctatus) in North America Are R. Berentsen, William C. Pitt, and Robert T. Sugihara CONTENTS General Ecology and Distribution......................................................................... -
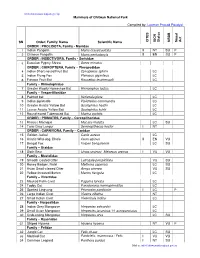
Mammals of Chitwan National Park Compiled By: Laxman Prasad Poudyal SN Order/ Family/ Name Scientific Name C IT E S IU C N S Ta
www.chitwannationalpark.gov.np Mammals of Chitwan National Park Compiled by: Laxman Prasad Poudyal SN Order/ Family/ Name Scientific Name CITES IUCN Status NRDB Nepal Act ORDER : PHOLIDOTA, Family - Manidae 1 Indian Pangolin Manis crassicaudata II NT SU P 2 Chinese Pangolin Manis pentadacyla II EN SU P ORDER : INSECTIVORA, Family - Soricidae 3 Eurasian Pygmy Shrew Sorex minutus ORDER : CHIROPTERA, Family – Pteropodidae 4 Indian Short-nosed Fruit Bat Cynopterus sphinx LC 5 Indian Flying Fox Pteropus giganteus LC 6 Fulvous Fruit Bat Rousettus leschenaulti LC Family – Rhinolophidae 7 Greater Woolly Horseshoe Bat Rhinolophus luctus LC Family – Vespertilionidae 8 Painted bat Kerivoula picta LC 9 Indian pipistrelle Pipistrellus coromandra LC 10 Greater Asiatic Yellow Bat Scotophilus heathi LC 11 Lesser Asiatic Yellow Bat Scotophilus kuhlii LC 12 Round-eared Tubenosed Bat Murina cyclotis LC ORDER : PRIMATES, Family – Cercopithecidae 13 Rhesus Macaque Macaca mulatta LC SU 14 Tarai Gray Langur Semnopithecus hector I NT ORDER : CARNIVORA, Family – Canidae 15 Golden Jackal Canis aureus LC 16 Asiatic Wild-dog, Dhole Cuon alpinus II EN VU 17 Bengal Fox Vulpes bengalensis LC SU Family – Ursidae 18 Sloth Bear Ursus ursinus/ Melursus ursinus I VU VU Family – Mustelidae 19 Smooth Coated Otter Lutrogale perspicillata VU SU 20 Honey Badger, Ratel Mellivora capensis LC SU 21 Asian Small-clawed Otter Aonyx cinerea VU SU 22 Yellow-throated Marten Martes flavigula LC Family – Viverridae 23 Masked Palm Civet Paguma larvata LC 24 Toddy Cat Paradoxurus hermaphroditus LC 25 Spotted Lingsang Prionodon pardicolor I LC P 26 Large Indian Civet Viverra zibetha NT 27 Small Indian Civet Viverricula indica LC Family - Herpestidae 28 Indian Grey Mongoose Herpestes edwardsii LC 29 Small Asian Mongoose Herpestes javanicus/ H. -

Herpetological Journal FULL PAPER
Volume 27 (April 2017), 201–216 Herpetological Journal FULL PAPER Published by the British Predation of Jamaican rock iguana Cyclura( collei) nests Herpetological Society by the invasive small Asian mongoose (Herpestes auropunctatus) and the conservation value of predator control Rick van Veen & Byron S. Wilson Department of Life Sciences, University of the West Indies, Mona 7, Kingston, Jamaica The introduced small Asian mongoose (Herpestes auropunctatus) has been widely implicated in extirpations and extinctions of island taxa. Recent studies and anecdotal observations suggest that the nests of terrestrial island species are particularly vulnerable to mongoose predation, yet quantitative data have remained scarce, even for species long assumed to be at risk from the mongoose. We monitored nests of the Critically Endangered Jamaican Rock Iguana (Cyclura collei) to determine nest fate, and augmented these observations with motion-activated camera trap images to document the predatory behaviour of the mongoose. Our data provide direct, quantitative evidence of high nest predation pressure attributable to the mongoose, and together with reported high rates of predation on hatchling and juvenile iguanas (also by the mongoose), support the original conclusion that the mongoose was responsible for the apparent lack of recruitment and the aging structure of the small population that was ‘re-discovered’ in 1990. Encouragingly however, our data also demonstrate a significant reduction in nest predation pressure within an experimental mongoose-removal area. Thus, our results indicate that otherwise catastrophic levels of nest loss (at or near 100%) can be ameliorated or even eliminated by removal trapping of the mongoose. We suggest that such targeted control efforts could also prove useful in safeguarding other threatened insular species with reproductive strategies that are notably vulnerable to mongoose predation (e.g., the incubation of eggs on or underground). -

Carnivore Hotspots in Peninsular Malaysia and Their Landscape
1 Carnivore hotspots in Peninsular Malaysia and their 2 landscape attributes 3 Shyamala Ratnayeke1*¶, Frank T. van Manen2¶, Gopalasamy Reuben Clements1&, Noor Azleen 4 Mohd Kulaimi3&, Stuart P. Sharp4& 5 1Department of Biological Sciences, Sunway University, Malaysia 6 7 2U.S. Geological Survey, Northern Rocky Mountain Science Center, Interagency Grizzly Bear Study Team, Bozeman, 8 MT 59715, USA 9 10 3Ex-Situ Conservation Division, Department of Wildlife and National Parks, Malaysia 11 12 4Lancaster Environment Centre, Lancaster University, Lancaster, LA1 4YQ, UK 13 14 15 16 *Corresponding author 17 Email: [email protected] 18 19 ¶SR and FTVM are joint senior authors 20 &These authors also contributed equally to this work 21 22 23 24 25 26 27 28 29 30 31 32 33 Disclaimer: This draft manuscript is distributed solely for purposes of scientific peer review. Its content is 34 deliberative and pre-decisional, so it must not be disclosed or released by reviewers. Because the 35 manuscript has not yet been approved for publication by the U.S. Geological Survey (USGS), it does not 36 represent any official USGS finding or policy. 37 Abstract 38 Mammalian carnivores play a vital role in ecosystem functioning. However, they are prone to 39 extinction because of low population densities and growth rates, large area requirements, and 40 high levels of persecution or exploitation. In tropical biodiversity hotspots such as Peninsular 41 Malaysia, rapid conversion of natural habitats threatens the persistence of this vulnerable 42 group of animals. Here, we carried out the first comprehensive literature review on 31 43 carnivore species reported to occur in Peninsular Malaysia and updated their probable 44 distribution.