Qualitative Shark Risk Assessment• 1
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Bibliography Database of Living/Fossil Sharks, Rays and Chimaeras (Chondrichthyes: Elasmobranchii, Holocephali) Papers of the Year 2016
www.shark-references.com Version 13.01.2017 Bibliography database of living/fossil sharks, rays and chimaeras (Chondrichthyes: Elasmobranchii, Holocephali) Papers of the year 2016 published by Jürgen Pollerspöck, Benediktinerring 34, 94569 Stephansposching, Germany and Nicolas Straube, Munich, Germany ISSN: 2195-6499 copyright by the authors 1 please inform us about missing papers: [email protected] www.shark-references.com Version 13.01.2017 Abstract: This paper contains a collection of 803 citations (no conference abstracts) on topics related to extant and extinct Chondrichthyes (sharks, rays, and chimaeras) as well as a list of Chondrichthyan species and hosted parasites newly described in 2016. The list is the result of regular queries in numerous journals, books and online publications. It provides a complete list of publication citations as well as a database report containing rearranged subsets of the list sorted by the keyword statistics, extant and extinct genera and species descriptions from the years 2000 to 2016, list of descriptions of extinct and extant species from 2016, parasitology, reproduction, distribution, diet, conservation, and taxonomy. The paper is intended to be consulted for information. In addition, we provide information on the geographic and depth distribution of newly described species, i.e. the type specimens from the year 1990- 2016 in a hot spot analysis. Please note that the content of this paper has been compiled to the best of our abilities based on current knowledge and practice, however, -

An Introduction to the Classification of Elasmobranchs
An introduction to the classification of elasmobranchs 17 Rekha J. Nair and P.U Zacharia Central Marine Fisheries Research Institute, Kochi-682 018 Introduction eyed, stomachless, deep-sea creatures that possess an upper jaw which is fused to its cranium (unlike in sharks). The term Elasmobranchs or chondrichthyans refers to the The great majority of the commercially important species of group of marine organisms with a skeleton made of cartilage. chondrichthyans are elasmobranchs. The latter are named They include sharks, skates, rays and chimaeras. These for their plated gills which communicate to the exterior by organisms are characterised by and differ from their sister 5–7 openings. In total, there are about 869+ extant species group of bony fishes in the characteristics like cartilaginous of elasmobranchs, with about 400+ of those being sharks skeleton, absence of swim bladders and presence of five and the rest skates and rays. Taxonomy is also perhaps to seven pairs of naked gill slits that are not covered by an infamously known for its constant, yet essential, revisions operculum. The chondrichthyans which are placed in Class of the relationships and identity of different organisms. Elasmobranchii are grouped into two main subdivisions Classification of elasmobranchs certainly does not evade this Holocephalii (Chimaeras or ratfishes and elephant fishes) process, and species are sometimes lumped in with other with three families and approximately 37 species inhabiting species, or renamed, or assigned to different families and deep cool waters; and the Elasmobranchii, which is a large, other taxonomic groupings. It is certain, however, that such diverse group (sharks, skates and rays) with representatives revisions will clarify our view of the taxonomy and phylogeny in all types of environments, from fresh waters to the bottom (evolutionary relationships) of elasmobranchs, leading to a of marine trenches and from polar regions to warm tropical better understanding of how these creatures evolved. -

NPOA Sharks Booklet.Indd
National Plan of Action for the Conservation and Management of Sharks (NPOA-Sharks) November 2013 South Africa Department of Agriculture, Forestry and Fisheries Private Bag X2, Rogge Bay, 8012 Tel: 021 402 3911 Fax: +27 21 402 3364 www.daff.gov.za Design and Layout: FNP Communications and Gerald van Tonder Photographs courtesy of: Department of Agriculture, Forestry and Fisheries (DAFF), Craig Smith, Charlene da Silva, Rob Tarr Foreword South Africa’s Exclusive Economic Zone is endowed with a rich variety of marine living South Africa is signatory to the Code of Conduct for Responsible Fisheries – voluntarily agreed to by members of the United Nations Food and Agriculture Organisation (FAO) – and, as such, is committed to the development and implementation of National Plans of Action (NPOAs) as adopted by the twenty-third session of the FAO Committee on Fisheries in February 1999 and endorsed by the FAO Council in June 1999. Seabirds – aimed at reducing incidental catch and promoting the conservation of seabirds Fisheries and now regularly conducts Ecological Risk Assessments for all the commercial practices. Acknowledging the importance of maintaining a healthy marine ecosystem and the possibility of major detrimental effects due to the disappearance of large predators, South from the list of harvestable species. In accordance with international recommendations, South Africa subsequently banned the landing of a number of susceptible shark species, including oceanic whitetip, silky, thresher and hammerhead sharks. improves monitoring efforts for foreign vessels discharging shark products in its ports. To ensure long-term sustainability of valuable, but biologically limited, shark resources The NPOA-Sharks presented here formalises and streamlines ongoing efforts to improve conservation and management of sharks caught in South African waters. -

Bulletin No 7.Qxd
Electrolux addisoni, a new genus and species of electric ray from the east coast of South Africa (Rajiformes: Torpedinoidei: Narkidae), with a review of torpedinoid taxonomy Leonard J.V. Compagno 1 and Phillip C. Heemstra 2 1 Shark Research Centre, Iziko-South African Museum, Cape Town, South Africa, e-mail [email protected] 2 South African Institute for Aquatic Biodiversity, Grahamstown, South Africa, e-mail [email protected] (Submitted 7 December 2006; accepted 23 February 2007) ABSTRACT . A new genus and species of sleeper ray, Electrolux addisoni (Family Narkidae), with two dorsal fins is described from two adult males (total lengths 50 and 52 cm) caught on a shallow reef off the east coast of South Africa. Electrolux is distinguished from other genera of Narkidae by its prominent spiracular papillae, the morphology of its nostrils, nasal curtain, mouth, jaws, chondrocranium, basibranchial skeleton, pectoral and pelvic girdles, and unique and complex colour pattern. It has higher vertebral, pectoral radial, tooth and intestinal valve counts than other narkids and reaches a greater size than all species with the possibly exception of Typhlonarke aysoni . Taxonomic definitions are provided for the electric rays, for the family Narkidae, and for Electrolux, as well as keys to families of electric rays and to the genera of Narkidae. The systematics of the narkid genus Heteronarce is reviewed and the genus validated. Members of the Narkidae may include the smallest, or at least the shortest, living chondrichthyans ( Temera hardwickii and an undescribed species of Narke ). Electrolux addisoni is a reef-dweller that eats polychaete worms and small crustaceans, and has been photographed and videotaped by divers while actively feeding in the daytime. -

© Iccat, 2007
A5 By-catch Species APPENDIX 5: BY-CATCH SPECIES A.5 By-catch species By-catch is the unintentional/incidental capture of non-target species during fishing operations. Different types of fisheries have different types and levels of by-catch, depending on the gear used, the time, area and depth fished, etc. Article IV of the Convention states: "the Commission shall be responsible for the study of the population of tuna and tuna-like fishes (the Scombriformes with the exception of Trichiuridae and Gempylidae and the genus Scomber) and such other species of fishes exploited in tuna fishing in the Convention area as are not under investigation by another international fishery organization". The following is a list of by-catch species recorded as being ever caught by any major tuna fishery in the Atlantic/Mediterranean. Note that the lists are qualitative and are not indicative of quantity or mortality. Thus, the presence of a species in the lists does not imply that it is caught in significant quantities, or that individuals that are caught necessarily die. Skates and rays Scientific names Common name Code LL GILL PS BB HARP TRAP OTHER Dasyatis centroura Roughtail stingray RDC X Dasyatis violacea Pelagic stingray PLS X X X X Manta birostris Manta ray RMB X X X Mobula hypostoma RMH X Mobula lucasana X Mobula mobular Devil ray RMM X X X X X Myliobatis aquila Common eagle ray MYL X X Pteuromylaeus bovinus Bull ray MPO X X Raja fullonica Shagreen ray RJF X Raja straeleni Spotted skate RFL X Rhinoptera spp Cownose ray X Torpedo nobiliana Torpedo -

BETTER DATA COLLECTION in SHARK FISHERIES LEARNING from PRACTICE Cover Image: Cacaodesign.It FAO Fisheries and Aquaculture Circular No
NFIF/C1227 (En) FAO Fisheries and Aquaculture Circular ISSN 2070-6065 BETTER DATA COLLECTION IN SHARK FISHERIES LEARNING FROM PRACTICE Cover image: cacaodesign.it FAO Fisheries and Aquaculture Circular No. 1227 NFIF/C1227 (En) BETTER DATA COLLECTION IN SHARK FISHERIES LEARNING FROM PRACTICE by Monica Barone Fishery Resources Consultant FAO Fisheries Department Rome, Italy and Kim Friedman Senior Fishery Resources Officer FAO Fisheries Department Rome, Italy FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS Rome, 2021 Required citation: FAO. 2021. Better data collection in shark fisheries – Learning from practice. FAO Fisheries and Aquaculture Circular No. 1227. Rome. https://doi.org/10.4060/cb5378en The designations employed and the presentation of material in this information product do not imply the expression of any opinion whatsoever on the part of the Food and Agriculture Organization of the United Nations (FAO) concerning the legal or development status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. The mention of specific companies or products of manufacturers, whether or not these have been patented, does not imply that these have been endorsed or recommended by FAO in preference to others of a similar nature that are not mentioned. The views expressed in this information product are those of the author(s) and do not necessarily reflect the views or policies of FAO. ISBN 978-92-5-134622-8 © FAO, 2021 Some rights reserved. This work is made available under the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 IGO licence (CC BY-NC-SA 3.0 IGO; https://creativecommons.org/licenses/by-nc-sa/3.0/igo/legalcode). -

Recreational Harvest of Sharks and Rays in Western Australia Is Only a Minor Component of the Total Harvest
Recreational Harvest of Sharks and Rays in Western Australia is only a Minor Component of the Total Harvest Matias Braccini ( [email protected] ) Department of Primary Industries and Regional Development Eva Lai Department of Primary Industries and Regional Development Karina Ryan Department of Primary Industries and Regional Development Stephen Taylor Department of Primary Industries and Regional Development Research Article Keywords: elasmobranch, conservation, management, sheries, catch reconstruction Posted Date: February 23rd, 2021 DOI: https://doi.org/10.21203/rs.3.rs-225321/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Page 1/20 Abstract Sharks and rays are of global conservation concern with an increasing number of species at risk of extinction, mostly attributed to overshing by commercial shing. Their recreational harvest is poorly known but it can be of similar magnitude to the commercial harvest in some regions. We quantied the recreational harvest of sharks and rays in Western Australia, a region with a marine coastline of > 20,000 km. We recorded 33 species/taxonomic groups but the harvest was dominated by dusky and bronze whalers, blacktip reef shark, gummy shark, Port Jackson shark, wobbegongs, and rays and skates. Most species caught were released (85% of all individuals), although gummy and whiskery sharks were typically retained. There was a clear latitudinal gradient of species composition with tropical and subtropical species of the genus Carcharhinus dominating in the north and temperate species of the families Triakidae, Carcharhinidae, Heterodontidae and Rajidae dominating in the south. The recreational harvest was negligible compared with commercial landings. -

Ground Sharks
click for previous page - v - TABLE OF CONTENTS Code Page 9. ORDER CARCHARHINIFORMES - GROUND SHARKS ....................................................................................... 251 9.1 FAMILY SCYLIORHINIDAE - Catsharks .................................................. SCYL ........................................... 253 Apristurus....................................................................................................... SCYL Aprist ................................ 257 A. atlanticus ..................................................................................... SCYL Aprist 1 ............................... 261 A. brunneus ...................................................................................... SCYL Aprist 2 ............................... 262 A. canutus ............................................................................................ SCYL Aprist 3 ............................... 263 A. herklotsi ........................................................................................ SCYL Aprist 4 ............................... 264 A. indicus ............................................................................................. SCYL Aprist 5 ............................... 265 A. investigatoris ................................................................................... SCYL Aprist 6 ............................... 267 A. japonicus ....................................................................................... SCYL Aprist 7 ............................... 268 -

New Zealand Fishes a Field Guide to Common Species Caught by Bottom, Midwater, and Surface Fishing Cover Photos: Top – Kingfish (Seriola Lalandi), Malcolm Francis
New Zealand fishes A field guide to common species caught by bottom, midwater, and surface fishing Cover photos: Top – Kingfish (Seriola lalandi), Malcolm Francis. Top left – Snapper (Chrysophrys auratus), Malcolm Francis. Centre – Catch of hoki (Macruronus novaezelandiae), Neil Bagley (NIWA). Bottom left – Jack mackerel (Trachurus sp.), Malcolm Francis. Bottom – Orange roughy (Hoplostethus atlanticus), NIWA. New Zealand fishes A field guide to common species caught by bottom, midwater, and surface fishing New Zealand Aquatic Environment and Biodiversity Report No: 208 Prepared for Fisheries New Zealand by P. J. McMillan M. P. Francis G. D. James L. J. Paul P. Marriott E. J. Mackay B. A. Wood D. W. Stevens L. H. Griggs S. J. Baird C. D. Roberts‡ A. L. Stewart‡ C. D. Struthers‡ J. E. Robbins NIWA, Private Bag 14901, Wellington 6241 ‡ Museum of New Zealand Te Papa Tongarewa, PO Box 467, Wellington, 6011Wellington ISSN 1176-9440 (print) ISSN 1179-6480 (online) ISBN 978-1-98-859425-5 (print) ISBN 978-1-98-859426-2 (online) 2019 Disclaimer While every effort was made to ensure the information in this publication is accurate, Fisheries New Zealand does not accept any responsibility or liability for error of fact, omission, interpretation or opinion that may be present, nor for the consequences of any decisions based on this information. Requests for further copies should be directed to: Publications Logistics Officer Ministry for Primary Industries PO Box 2526 WELLINGTON 6140 Email: [email protected] Telephone: 0800 00 83 33 Facsimile: 04-894 0300 This publication is also available on the Ministry for Primary Industries website at http://www.mpi.govt.nz/news-and-resources/publications/ A higher resolution (larger) PDF of this guide is also available by application to: [email protected] Citation: McMillan, P.J.; Francis, M.P.; James, G.D.; Paul, L.J.; Marriott, P.; Mackay, E.; Wood, B.A.; Stevens, D.W.; Griggs, L.H.; Baird, S.J.; Roberts, C.D.; Stewart, A.L.; Struthers, C.D.; Robbins, J.E. -

First Records of the False Catshark, Pseudotriakis Microdon Capello, 1868, from the Waters of Eastern Australia and Indonesia
VOLUME 51 PART 2 MEMOIRS OF THE QUEENSLAND MUSEUM BRISBANE 31 DECEMBER 2005 © Queensland Museum PO Box 3300, South Brisbane 4101, Australia Phone 06 7 3840 7555 Fax 06 7 3846 1226 Email [email protected] Website www.qmuseum.qld.gov.au National Library of Australia card number ISSN 0079-8835 NOTE Papers published in this volume and in all previous volumes of the Memoirs of the Queensland Museum may be reproduced for scientific research, individual study or other educational purposes. Properly acknowledged quotations may be made but queries regarding the republication of any papers should be addressed to the Director. Copies of the journal can be purchased from the Queensland Museum Shop. A Guide to Authors is displayed at the Queensland Museum web site www.qmuseum.qld.gov.au/resources/resourcewelcome.html A Queensland Government Project Typeset at the Queensland Museum FIRST RECORDS OF THE FALSE CATSHARK, PSEUDOTRIAKIS MICRODON CAPELLO, 1868, FROM THE WATERS OF EASTERN AUSTRALIA AND INDONESIA PETER M. KYNE, JEFFREY W. JOHNSON, WILLIAM T. WHITE AND MICHAEL B. BENNETT Kyne, P.M., Johnson, J.W., White, W.T. & Bennett, M.B. 2005 12 31: First records of the false catshark, Pseudotriakis microdon Capello, 1868, from the waters of eastern Australia and Indonesia. Memoirs of the Queensland Museum 51(2): 525-530. Brisbane. ISSN 0079-8835. A new specimen of a rare deepwater chondrichthyan, the false catshark Pseudotriakis microdon Capello, 1868 is documented from the Coral Sea. This represents the first record of the species from off the east coast of Australia and only the second from Australian waters. -
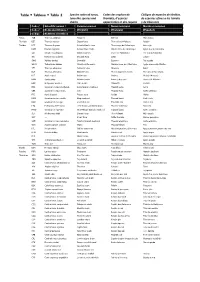
Table Tableau Tabla 2
Table Tableau Tabla 2 Species codes of tunas, Codes des espèces de Códigos de especies de túnidos, tuna‐like species and thonidés, d’espèces de especies afines a los túnidos sharks apparentées et des requins y de tiburones Code / Scientific names / Common names Noms communs Nombres comunes Code / Noms sientifiques / (English) (Français) (Español) Código Nombres científicos Tunas ALB Thunnus alalunga Albacore Germon Atún blanco Thonidés BET Thunnus obesus Bigeye tuna Thon obèse(=Patudo) Patudo Túnidos BFT Thunnus thynnus Atlantic bluefin tuna Thon rouge de l’atlantique Atún rojo BUM Makaira nigricans Atlantic blue marlin Makaire bleu de l'Atlantique Aguja azul del Atlántico SAI Istiophorus albicans Atlantic sailfish Voilier de l'Atlantique Pez vela del Atlántico SKJ Katsuwonus pelamis Skipjack tuna Listao Listado SWO Xiphias gladius Swordfish Espadon Pez espada WHM Tetrapturus albidus Atlantic white marlin Makaire blanc de l'Atlantique Aguja blanca del Atlántico YFT Thunnus albacares Yellowfin tuna Albacore Rabil BLF Thunnus atlanticus Blackfin tuna Thon à nageoires noires Atún des aletas negras BLT Auxis rochei Bullet tuna Bonitou Melva(=Melvera) BON Sarda sarda Atlantic bonito Bonite à dos rayé Bonito del Atlántico BOP Orcynopsis unicolor Plain bonito Palomette Tasarte BRS Scomberomorus brasiliensis Serra Spanish mackerel Thazard serra Serra CER Scomberomorus regalis Cero Thazard franc Carite chinigua FRI Auxis thazard Frigate tuna Auxide Melva KGM Scomberomorus cavalla King mackerel Thazard barré Carite lucio KGX Scomberomorus spp -

Identification Guide to the Deep-Sea Cartilaginous Fishes Of
Identification guide to the deep–sea cartilaginous fishes of the Southeastern Atlantic Ocean FAO. 2015. Identification guide to the deep–sea cartilaginous fishes of the Southeastern Atlantic Ocean. FishFinder Programme, by Ebert, D.A. and Mostarda, E., Rome, Italy. Supervision: Merete Tandstad, Jessica Sanders (FAO, Rome) Technical editor: Edoardo Mostarda (FAO, Rome) Colour illustrations, cover and graphic design: Emanuela D’Antoni (FAO, Rome) This guide was prepared under the “FAO Deep–sea Fisheries Programme” thanks to a generous funding from the Government of Norway (Support to the implementation of the International Guidelines on the Management of Deep-Sea Fisheries in the High Seas project) for the purpose of assisting states, institutions, the fishing industry and RFMO/As in the implementation of FAO International Guidelines for the Management of Deep-sea Fisheries in the High Seas. It was developed in close collaboration with the FishFinder Programme of the Marine and Inland Fisheries Branch, Fisheries Department, Food and Agriculture Organization of the United Nations (FAO). The present guide covers the deep–sea Southeastern Atlantic Ocean and that portion of Southwestern Indian Ocean from 18°42’E to 30°00’E (FAO Fishing Area 47). It includes a selection of cartilaginous fish species of major, moderate and minor importance to fisheries as well as those of doubtful or potential use to fisheries. It also covers those little known species that may be of research, educational, and ecological importance. In this region, the deep–sea chondrichthyan fauna is currently represented by 50 shark, 20 batoid and 8 chimaera species. This guide includes full species accounts for 37 shark, 9 batoid and 4 chimaera species selected as being the more difficult to identify and/or commonly caught.