2016 Dentallib P C Anila Namboodiripad
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

ADEA Compendium of Curriculum Guidelines Allied Dental Education
ADEA Compendium of Curriculum Guidelines (Revised Edition) Allied Dental Education Programs May 2015–2016 ADEA Compendium of Curriculum Guidelines for Allied Dental Education Programs May 2015–2016 CONTENTS INTRODUCTION .................................................................................................. 2 ACKNOWLEDGEMENTS ...................................................................................... 5 DENTAL HYGIENE CURRICULUM GUIDELINES ................................................... 8 Clinical and Preclinical Dental Hygiene ........................................................... 8 Community Dental Health ............................................................................. 21 Dental Materials ............................................................................................ 31 Medical Emergencies .................................................................................... 53 Nutrition ........................................................................................................ 60 Oral Anatomy and Histo-embryology ............................................................ 70 Oral Pathology............................................................................................... 97 Periodontology ............................................................................................ 109 Pharmacology .............................................................................................. 125 Research for Dental Hygiene Education ..................................................... -

Dantal College Inner Pages Vol-7 Issue-2.Cdr
JADCH ISSN 0976-2256 E-ISSN: 2249-6653 The journal is indexed with ‘Indian Science Abstract’ (ISA) (Published by National Science Library), www.ebscohost.com, www.indianjournals.com JADCH is available (full text) online: Website- www.adc.org.in/html/viewJournal.php This journal is an official publication of Ahmedabad Dental College and Hospital, published bi-annually in the month of March and September. The journal is printed on ACID FREE paper. Editor - in - Chief Dr. Darshana Shah Co - Editor Dr. Harsh Shah Editorial Board: DENTISTRY TODAY... The last decade has witnessed the most rapid advances in Dr. Mihir Shah the field of oral andmaxillofacial radiology. With the advent Dr. Vijay Bhaskar and acceptance of CBCT (3D) imaging invarious fields of dentistry; the dentists today are taking more accurate and Dr. Monali Chalishazar informeddecisions regarding complicated patients and their treatment planning. However, today’spatients demand more Dr. A. R. Chaudhary and more comfort and less intraoperative time; even if it comes Dr. Neha Vyas athigher treatment cost. With ever increasing patient affordability as well as expectations, the least should be left to Dr. Sonali Mahadevia dental surgeon’s imaginations. Dr. Shraddha Chokshi Welcome to the era of 3D printing; the next step after 3D Dr. Bhavin Dudhia imaging. With 3D printedmodels on hand, the dentist can actually perform a mock surgery on a model, whichrepresents Dr. Mahadev Desai the patient accurately in three dimensions; and hence reduces the intraoperative time. 3D printing, also called additive Dr. Darshit Dalal manufacturing; creates a physicalobject by layer by layer deposition of material. This technology can be helpful inpreparing surgical stents for implant placements, models for oral and maxillofacialtrauma and pathology cases, prosthetic rehabilitation cases, complicated endodontic casesas well as for orthodontic appliances. -

General Dentistry
QUINTESSENCE INTERNATIONAL GENERAL DENTISTRY Adrian Kasaj Root resective procedures vs implant therapy in the management of furcation-involved molars Adrian Kasaj, PD Dr med dent1 Therapeutic decision making and successful treatment of fur- ment, the clinician is increasingly confronted with the dilemma cation-involved molars has been a challenge for many clin- of whether to treat a furcated molar by traditional root resec- icians. Over recent decades, several techniques have been tive techniques or to extract the tooth and replace it with a advocated in the treatment of furcated molar teeth, including dental implant. This article reviews the outcomes of root resec- nonsurgical periodontal therapy, regenerative therapy, and tive therapy for the management of furcation-involved multi- resective surgical procedures. Today, root resection is consid- rooted teeth and discusses treatment alternatives including ered a relevant treatment modality in the management of fur- implant therapy. Treatment guidelines for root resective thera- cation-involved multirooted molars. However, root resective py, along with advantages and limitations, are presented to procedures are very technique-sensitive and require a high help the clinician in the decision-making process. level of periodontal, endodontic, and restorative expertise. (Quintessence Int 2014;45:521–529; doi: 10.3290/j.qi.a31806) Given the high documented success rates of implant treat- Key words: furcation involvement, furcations, molar, periodontal disease, root resection The management and long-term retention of furcated teeth without furcation involvement.3,4 Even with a molar teeth has always been a challenge for clinicians. surgical approach selected to improve access for root Furcation involvement is defined as interradicular bone surface debridement, complete calculus removal in the resorption and attachment loss in multirooted teeth furcation area is rare.5 The compromised results in fur- caused by periodontal disease. -

Endodontic Management of Central Incisor Associated with Large Periapical Lesion and Fused Supernumerary Root: a Conservative Approach
Restor Dent Endod. 2018 Nov;43(4):e44 https://doi.org/10.5395/rde.2018.43.e44 pISSN 2234-7658·eISSN 2234-7666 Case Report Endodontic management of central incisor associated with large periapical lesion and fused supernumerary root: a conservative approach Gautam P. Badole ,1* Pratima R. Shenoi ,1 Ameya Parlikar 2 1Department of Conservative Dentistry & Endodontics, VSPM's Dental College & Research Center, Nagpur, MH, India 2Department of Conservative Dentistry & Endodontics, Rangoonwala Dental College and Research Center, Pune, MH, India Received: Mar 19, 2018 ABSTRACT Accepted: Aug 21, 2018 Badole GP, Shenoi PR, Parlikar A Fusion and gemination are developmental anomalies of teeth that may require endodontic treatment. Fusion may cause various clinical problems related to esthetics, tooth spacing, and *Correspondence to other periodontal complications. Additional diagnostic tools are required for the diagnosis and Gautam P. Badole, MDS Reader, Department of Conservative Dentistry the treatment planning of fused tooth. The present case report describes a case of unilateral & Endodontics, VSPM's Dental College & fusion of a supernumerary root to an upper permanent central incisor with large periapical Research Center, Hingna Road, Digdoh Hills, lesion in which a conservative approach was used without extraction of supernumerary tooth Nagpur, MH 440019, India. and obturated with mineral trioxide aggregate to reach a favorable outcome. E-mail: [email protected] Keywords: Cone-beam computed tomography; Fused teeth; Mineral trioxide aggregate; Copyright © 2018. The Korean Academy of Supernumerary tooth Conservative Dentistry This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (https:// INTRODUCTION creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any Developmental tooth anomalies are deviation from the normal appearance in color, medium, provided the original work is properly shape, size and number of teeth. -

COMPENDIUM of CURRICULUM GUIDELINES (Revised Edition)
COMPENDIUM OF CURRICULUM GUIDELINES (Revised Edition) ALLIED DENTAL EDUCATION PROGRAMS February 2005 TABLE OF CONTENTS Introduction…………………………………………………………………………….3 Acknowledgments…………………………………………………………………….6 DENTAL HYGIENE CURRICULUM GUIDELINES Clinical Dental Hygiene……………………………………………………………...10 Community Dental Health…………………………………………………………...21 Dental Materials……………………………………………………………………….28 Medical Emergencies……………………………………………………………….. 49 Nutrition……………………………………………………………………………….. 55 Oral and Facial Anatomy…………………………………………………………….65 Oral Pathology…………………………………………………………………………88 Periodontology……………………………………………………………..................98 Pharmacology…………………………………………………………………………110 Research………………………………………………………………………………..123 Special Needs Patients………………………………………………………………129 DENTAL ASSISTING CURRICULUM GUIDELINES Pathology……………………………………………………………………………….137 Preclinical Dental Assisting…………………………………………………………145 DENTAL HYGIENE AND DENTAL ASSISTING CURRICULUM GUIDELINES Dental Radiography……………………………………………………………………152 Radiation Use Guidelines……………………………………………………………..173 Clinical Radiology………………………………………………………………………178 Ethics and Professionalism…………………………………………………………..184 2 INTRODUCTION This document is a revision of curriculum guidelines that were developed for allied dental education programs between 1984 and 1994. It does not include all content areas that could be found in an allied dental education program. Most of the guidelines are for dental hygiene with some for dental assisting. Unfortunately, no guidelines were developed during this time -

Poster Communications
1113-5181/19/27.1/94-120 ODONTOLOGÍA PEDIÁTRICA ODONTOL PEDIÁTR (Madrid) COPYRIGHT © 2019 SEOP Y ARÁN EDICIONES, S. L. Vol. 27, N.º 1, pp. 94-120, 2019 Poster communications RESEARCH STUDIES 10. CHANGES IN ORAL HEALTH-RELATED QUALITY OF LIFE WHEN ASSOCIATED WITH THE TYPE OF CLEFT LIP AND/OR PALATE IN SURGICALLY TREATED CHILDREN 8. EFFECT OF INHALED MEDICATION ON THE López Ramos, R.P.1; Abanto, J.2; Blanco, D.3; Torres, ORAL HEALTH OF ASTHMATIC PATIENTS G.4; Pajuelo, M.3 1 1 2 3 4 Faculty of Public Health and Administration. Peruvian Pinto, V. ; Menor, A. ; Gallegos, L. ; Martínez, E. 2 1Clínica Pinto. Burgos. 2Centro de Salud de Coria. Cáceres. University of Cayetano Heredia. Lima, Peru. Department 3 4 of Pediatric Dentistry. University of São Paulo. São Alfonso X El Sabio University. Madrid. Complutense 3 University of Madrid. Madrid Paulo, Brasil. Faculy of Sciences and Philosophy. Peruvian University Cayetano Heredia. Lima, Peru. 4 Introduction and objectives: Currently, and with increasing Postgraduate course on Pediatric Dentistry. Faculty of frequency, respiratory disorders are affecting a large percen- Pediatric Dentistry. National University of San Marcos. tage of the child population. The literature reviewed in this Lima, Peru research project shows that the use of inhaled medication for respiratory conditions is related to adverse reactions such as Introduction and objectives: The most common cranio- erosion, dental caries, gingivitis, halitosis or xerostomia. The facial malformation in children is the cleft lip and/or palate objective of the present study was to evaluate the relations- and the treatment is multidisciplinary. -

TV's COMPLIED NDBE QUESTIONS 2013‐2015
TV’s COMPLIED NDBE QUESTIONS 2013‐2015 https://quizlet.com/87020251/stuff‐to‐remember‐flash‐cards/ Compiled + 2015 + notes = purple + Highlighted FUNGI *A chlamydospore is the thick‐walled big resting spore of several kinds of fungi, including Ascomycota such as Candida and Basidiomycota such as Panus. It is the life‐stage which survives in unfavourable conditions, such as dry or hot seasons. 1. Organisms that exhibit dimorphism and grow on Sabouraud's medium (low pH): Fungi 2. Type of agar used for most fungi? Sabouraud agar 3. Organism that causes athletes foot (tinea pedis) Trichophyton 4. Which fungal infection leads to superficial skin disease? Trichophyton ‐ Epidermophyton & Microsporum cause dermatophytosis. Tx w/ Griseofulvin 5. Which fungus causes cerebral/brain infarct? a. Cryptococcus may spread into the meninges and cause Cryptococcal Meningitis b. Aspergillus causes aspergilloma “fungus ball” in the lungs causing pulmonary infection in ppl with AIDS or have undergone organ transplant c. Mucormycosis Pt’s w/ diabetic ketoacidosis, burns, or leukemia are particularly susceptible. It results in black, dead tissue in the nasal cavity and blocks the blood supply to the brain. ‐ Mucormycosis is found in blood vessels (endothelium) & is often related to diabetic pts 6. Aflatoxin is what produced by what fungus? Aspergillus 7. Cell immunity is most important for? Intracellular parasiteow 8. Which one can be seen as an intracellular organism? Histoplasmosis ‐ In infected tissues, yeast cells of Histoplasmosis are found within macrophages. 9. Disseminated fungi? Histoplasmosis (infection by a fungus found in the droppings of birds and bats in humid areas. It is not serious if confined to the lungs but can be fatal if spread throughout the body) 10. -

THE YOUNG and the OLD: NORMAL and VARIATIONS from NORMAL Judy Rochette DVM, FAVD, Dipl AVDC
THE YOUNG AND THE OLD: NORMAL AND VARIATIONS FROM NORMAL Judy Rochette DVM, FAVD, Dipl AVDC The majority of notable findings in the oral cavity of the young pet are related to its formation and development, as well as the development and eruption of teeth, whereas senior pet variations reflect the gradual aging of the dental hard tissues and their supporting structures. The head, face, and oral cavity are some of the first structures to manifest in the developing embryo. The upper face forms from the neural tube while the lower face forms from branchial arches. The oral mucosa and upper alimentary tract form from the ectodermal layers. The mesenchymal layer provides the cells which will become subcutaneous tissues and the bone and supporting apparatus for the teeth. The teeth themselves are both ectodermal and mesodermal in origin. Any phenotypic variation from the "wild type" of head, or any genetic anomaly which affects the ectoderm or mesodermal tissues will likely have an effect on the oral cavity. Some of these anomalies have been intentionally introduced to create new "breeds". BRACHYCEPHALIC SYNDROME Brachycephalic syndrome is a set of dysfunctional anatomical airway variations familiar to veterinarians who deal with Bulldogs, Pugs and Boston Terriers but this syndrome is also a problem in Persian, Himalayan and Burmese cats. Stenotic nares, an elongated soft palate, hypoplastic trachea and everted laryngeal saccules can all inhibit respiration. Mouth breathing, stertor, exercise intolerance and collapse after exercise can be seen. Early surgical intervention to open the nares and shorten the palate will often arrest the development of everted saccules, lower respiratory tract inflammation and cardiac stress. -
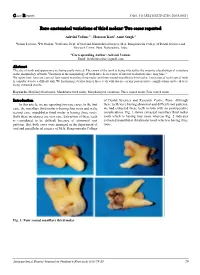
Rare Anatomical Variations of Third Molars: Two Cases Reported
Case Report DOI: 10.18231/2278-3784.2018.0021 Rare anatomical variations of third molars: Two cases reported Ashvini Vadane1,*, Hassaan Kazi2, Amit Sangle3 1Senior Lecturer, 2PG Student, 3Professor, Dept. of Oral and Maxillofacial Surgery, M.A. Rangoonwala College of Dental Sciences and Research Centre, Pune, Maharashtra, India *Corresponding Author: Ashvini Vadane Email: [email protected] Abstract The size of tooth and appearance are being easily noticed. The crown of the tooth is being affected by the majority of pathological variations in the morphology of tooth. Variations in the morphology of tooth have been a topic of interest to dentists since long time.1 We report here, two rare cases of four-rooted maxillary third molar and three-rooted mandibular third molar. Extraction of such type of teeth is considered to be a difficult task. We had managed extraction of these teeth with absence of any postoperative complications and teeth were being extracted in-toto. Keywords: Maxillary third molar, Mandibular third molar, Morphological variations, Three rooted molar, Four rooted molar. Introduction of Dental Sciences and Research Centre, Pune. Although In this article, we are reporting two rare cases .In the first these teeth were having abnormal and difficult root patterns, case, the maxillary third molar is having four roots and in the we had extracted these teeth in-toto with no postoperative second case, mandibular third molar is having three roots. complications. Fig. 1 shows extracted maxillary third molar Both these incidences are very rare. Extraction of these teeth tooth which is having four roots whereas Fig. 2 indicates is considered to be difficult because of abnormal root extracted mandibular third molar tooth which is having three patterns. -

What the General Dental Practitioner Should Know About Cone Beam Com- Puted Tomograph Technology
What the general dental practitioner should know about cone beam com- puted tomograph technology Magdalena Marinescu Gava1 1 D.D.S. Pirkanmaa Hospital District, Regional Imaging Centre, Tampere, Finland. Abstract Ionising radiation is used in health care for the diagnosis of diseases, for prevention (such as screening for breast can- cer) and for treatment (radiotherapy). The aim of radiation protection is to ensure that radiation is used safely and that exposure of the patient is kept to a minimum. The principles of radiation protection are based on the recommendations of the International Commission on Radiological Protection (ICRP). To be acceptable, the use of ionising radiation must be justified, optimal, and limited. Dental radiography has been used since the beginning of radiology and now accounts for nearly one-third of the total number of radiological examinations in the European Union. Pantomography is widely used because in one exposure it records the jaws, temporomandibular joints, maxillary sinuses, and dentition. However, when this technique is used, superimposition of structures in the dentomaxillofacial area has been a limitation in mak- ing reliable diagnoses due to the complexity of the anatomy in this region. These shortcomings have led to development of three-dimensional techniques. One of these techniques, cone-beam computed tomography (CBCT), is now available for use in everyday general practice. The aim of this paper is to present CBCT’s advantages and limitations when com- pared with alternative imaging techniques and to stress how it may be used safely in general dental practice. Key Words: Medical Subjects, Radiation Protection, Dentistry, Cone-Beam Computed Tomography (CBCT) Introduction the complicated human anatomy. -

Description Concept ID Synonyms Definition
Description Concept ID Synonyms Definition Category ABNORMALITIES OF TEETH 426390 Subcategory Cementum Defect 399115 Cementum aplasia 346218 Absence or paucity of cellular cementum (seen in hypophosphatasia) Cementum hypoplasia 180000 Hypocementosis Disturbance in structure of cementum, often seen in Juvenile periodontitis Florid cemento-osseous dysplasia 958771 Familial multiple cementoma; Florid osseous dysplasia Diffuse, multifocal cementosseous dysplasia Hypercementosis (Cementation 901056 Cementation hyperplasia; Cementosis; Cementum An idiopathic, non-neoplastic condition characterized by the excessive hyperplasia) hyperplasia buildup of normal cementum (calcified tissue) on the roots of one or more teeth Hypophosphatasia 976620 Hypophosphatasia mild; Phosphoethanol-aminuria Cementum defect; Autosomal recessive hereditary disease characterized by deficiency of alkaline phosphatase Odontohypophosphatasia 976622 Hypophosphatasia in which dental findings are the predominant manifestations of the disease Pulp sclerosis 179199 Dentin sclerosis Dentinal reaction to aging OR mild irritation Subcategory Dentin Defect 515523 Dentinogenesis imperfecta (Shell Teeth) 856459 Dentin, Hereditary Opalescent; Shell Teeth Dentin Defect; Autosomal dominant genetic disorder of tooth development Dentinogenesis Imperfecta - Shield I 977473 Dentin, Hereditary Opalescent; Shell Teeth Dentin Defect; Autosomal dominant genetic disorder of tooth development Dentinogenesis Imperfecta - Shield II 976722 Dentin, Hereditary Opalescent; Shell Teeth Dentin Defect; -

Dental and Temporomandibular Joint Pathology of the Walrus (Odobenus Rosmarus)
UC Davis UC Davis Previously Published Works Title Dental and Temporomandibular Joint Pathology of the Walrus (Odobenus rosmarus). Permalink https://escholarship.org/uc/item/05z8k86z Journal Journal of comparative pathology, 155(2-3) ISSN 0021-9975 Authors Winer, JN Arzi, B Leale, DM et al. Publication Date 2016-08-13 DOI 10.1016/j.jcpa.2016.07.005 Peer reviewed eScholarship.org Powered by the California Digital Library University of California J. Comp. Path. 2016, Vol. -,1e12 Available online at www.sciencedirect.com ScienceDirect www.elsevier.com/locate/jcpa DISEASE IN WILDLIFE OR EXOTIC SPECIES Dental and Temporomandibular Joint Pathology of the Walrus (Odobenus rosmarus) J. N. Winer*, B. Arzi†, D. M. Leale†,P.H.Kass‡ and F. J. M. Verstraete† *William R. Pritchard Veterinary Medical Teaching Hospital, † Department of Surgical and Radiological Sciences and ‡ Department of Population Health and Reproduction, School of Veterinary Medicine, University of California, Davis, CA, USA Summary Maxillae and/or mandibles from 76 walruses (Odobenus rosmarus) were examined macroscopically according to predefined criteria. The museum specimens were acquired between 1932 and 2014. Forty-five specimens (59.2%) were from male animals, 29 (38.2%) from female animals and two (2.6%) from animals of unknown sex, with 58 adults (76.3%) and 18 young adults (23.7%) included in this study. The number of teeth available for examination was 830 (33.6%); 18.5% of teeth were absent artefactually, 3.3% were deemed to be absent due to acquired tooth loss and 44.5% were absent congenitally. The theoretical complete dental formula was confirmed to be I 3/3, C 1/1, P 4/3, M 2/2, while the most probable dental formula is I 1/0, C 1/1, P 3/3, M 0/0; none of the specimens in this study possessed a full complement of theoretically possible teeth.