Crystal Darter Status Assessment Report
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Species Status Assessment Report for the Barrens Darter (Etheostoma Forbesi)
Species Status Assessment Report for the Barrens Darter (Etheostoma forbesi) Version 2.0 Acknowledgements: This Species Status Assessment would not have been possible without the research and assistance of Dr. Richard Harrington, Yale University Department of Ecology and Evolutionary Biology, Dr. Hayden Mattingly and his students, Tennessee Tech University School of Environmental Studies, Dr. John Johansen, Austin Peay State University Department of Biology, and Mark Thurman, Tennessee Wildlife Resources Agency. 1 TABLE OF CONTENTS Chapter 1: Introduction ............................................................................................................... 3 Chapter 2: Biology and Life History ........................................................................................... 4 Taxonomy ................................................................................................................................ 4 Genetic Diversity ..................................................................................................................... 5 Morphological Description ...................................................................................................... 5 Habitat ..................................................................................................................................... 6 Lifecycle .................................................................................................................................. 7 Population Needs .................................................................................................................... -

Fish Inventory at Stones River National Battlefield
Fish Inventory at Stones River National Battlefield Submitted to: Department of the Interior National Park Service Cumberland Piedmont Network By Dennis Mullen Professor of Biology Department of Biology Middle Tennessee State University Murfreesboro, TN 37132 September 2006 Striped Shiner (Luxilus chrysocephalus) – nuptial male From Lytle Creek at Fortress Rosecrans Photograph by D. Mullen Table of Contents List of Tables……………………………………………………………………….iii List of Figures………………………………………………………………………iv List of Appendices…………………………………………………………………..v Executive Summary…………………………………………………………………1 Introduction…………………………………………………………………...……..2 Methods……………………………………………………………………………...3 Results……………………………………………………………………………….7 Discussion………………………………………………………………………….10 Conclusions………………………………………………………………………...14 Literature Cited…………………………………………………………………….15 ii List of Tables Table1: Location and physical characteristics (during September 2006, and only for the riverine sites) of sample sites for the STRI fish inventory………………………………17 Table 2: Biotic Integrity classes used in assessing fish communities along with general descriptions of their attributes (Karr et al. 1986) ………………………………………18 Table 3: List of fishes potentially occurring in aquatic habitats in and around Stones River National Battlefield………………………………………………………………..19 Table 4: Fish species list (by site) of aquatic habitats at STRI (October 2004 – August 2006). MF = McFadden’s Ford, KP = King Pond, RB = Redoubt Brannan, UP = Unnamed Pond at Redoubt Brannan, LC = Lytle Creek at Fortress Rosecrans……...….22 Table 5: Fish Species Richness estimates for the 3 riverine reaches of STRI and a composite estimate for STRI as a whole…………………………………………………24 Table 6: Index of Biotic Integrity (IBI) scores for three stream reaches at Stones River National Battlefield during August 2005………………………………………………...25 Table 7: Temperature and water chemistry of four of the STRI sample sites for each sampling date…………………………………………………………………………….26 Table 8 : Total length estimates of specific habitat types at each riverine sample site. -

C:\Fish\Eastern Sand Darter Sa.Wpd
EASTERN SAND DARTER STATUS ASSESSMENT Prepared by: David Grandmaison and Joseph Mayasich Natural Resources Research Institute University of Minnesota 5013 Miller Trunk Highway Duluth, MN 55811-1442 and David Etnier Ecology and Evolutionary Biology University of Tennessee 569 Dabney Hall Knoxville, TN 37996-1610 Prepared for: U.S. Fish and Wildlife Service Region 3 1 Federal Drive Fort Snelling, MN 55111 January 2004 NRRI Technical Report No. NRRI/TR-2003/40 DISCLAIMER This document is a compilation of biological data and a description of past, present, and likely future threats to the eastern sand darter, Ammocrypta pellucida (Agassiz). It does not represent a decision by the U.S. Fish and Wildlife Service (Service) on whether this taxon should be designated as a candidate species for listing as threatened or endangered under the Federal Endangered Species Act. That decision will be made by the Service after reviewing this document; other relevant biological and threat data not included herein; and all relevant laws, regulations, and policies. The result of the decision will be posted on the Service's Region 3 Web site (refer to: http://midwest.fws.gov/eco_serv/endangrd/lists/concern.html). If designated as a candidate species, the taxon will subsequently be added to the Service's candidate species list that is periodically published in the Federal Register and posted on the World Wide Web (refer to: http://endangered.fws.gov/wildlife.html). Even if the taxon does not warrant candidate status it should benefit from the conservation recommendations that are contained in this document. ii TABLE OF CONTENTS DISCLAIMER................................................................... -

Best Management Practices
Crystal Darter Crystallaria asprella Guidelines for Landowners Using Conservation Practices Missouri Department of Conservation Photo Credit: Missouri Department of Conservation Common name ▪ Crystal Darter Recommendations Scientific name ▪ Crystallaria asprella As a species that prefers clean streams, crystal State status ▪ Endangered darters may act as indicators of a healthy Federal status ▪ None ecosystem. Protecting and restoring streams for the crystal darter will also benefit other aquatic species. Ecology Efforts should be made to ensure our waterways are Crystal darters have a large historic range, healthy through protection and/or restoration of stretching from river basins in West Virginia west to habitat for this and other aquatic species. Missouri and from Minnesota south to the Gulf of Mexico. In east-central to southeastern Missouri, Avoid constructing stream crossings. If they inhabit open channels of large, clear streams unavoidable, culverts and crossings should be and ditches with low to moderate gradients and long constructed with the same bottom elevation as the stretches of silt-free sand and small gravel existing streambed to avoid restricting flow and substrate. They prefer streams with strong current obstructing fish passage. and water depths of about 3 feet. The biology of this darter in Missouri is poorly known. Studies Bank stabilization materials should consist only of suggest that darters may bury themselves in the rock, clean broken concrete or similar materials free sand during the day and become active at night. of pollutants, silt and extraneous debris including Crystal darters forage for mainly aquatic insects, exposed rebar. Erosion and sediment controls especially midges, mosquitoes, blackflies and should be implemented, maintained and monitored caddisflies. -

Environmental Sensitivity Index Guidelines Version 2.0
NOAA Technical Memorandum NOS ORCA 115 Environmental Sensitivity Index Guidelines Version 2.0 October 1997 Seattle, Washington noaa NATIONAL OCEANIC AND ATMOSPHERIC ADMINISTRATION National Ocean Service Office of Ocean Resources Conservation and Assessment National Ocean Service National Oceanic and Atmospheric Administration U.S. Department of Commerce The Office of Ocean Resources Conservation and Assessment (ORCA) provides decisionmakers comprehensive, scientific information on characteristics of the oceans, coastal areas, and estuaries of the United States of America. The information ranges from strategic, national assessments of coastal and estuarine environmental quality to real-time information for navigation or hazardous materials spill response. Through its National Status and Trends (NS&T) Program, ORCA uses uniform techniques to monitor toxic chemical contamination of bottom-feeding fish, mussels and oysters, and sediments at about 300 locations throughout the United States. A related NS&T Program of directed research examines the relationships between contaminant exposure and indicators of biological responses in fish and shellfish. Through the Hazardous Materials Response and Assessment Division (HAZMAT) Scientific Support Coordination program, ORCA provides critical scientific support for planning and responding to spills of oil or hazardous materials into coastal environments. Technical guidance includes spill trajectory predictions, chemical hazard analyses, and assessments of the sensitivity of marine and estuarine environments to spills. To fulfill the responsibilities of the Secretary of Commerce as a trustee for living marine resources, HAZMAT’s Coastal Resource Coordination program provides technical support to the U.S. Environmental Protection Agency during all phases of the remedial process to protect the environment and restore natural resources at hundreds of waste sites each year. -

Endangered Species
FEATURE: ENDANGERED SPECIES Conservation Status of Imperiled North American Freshwater and Diadromous Fishes ABSTRACT: This is the third compilation of imperiled (i.e., endangered, threatened, vulnerable) plus extinct freshwater and diadromous fishes of North America prepared by the American Fisheries Society’s Endangered Species Committee. Since the last revision in 1989, imperilment of inland fishes has increased substantially. This list includes 700 extant taxa representing 133 genera and 36 families, a 92% increase over the 364 listed in 1989. The increase reflects the addition of distinct populations, previously non-imperiled fishes, and recently described or discovered taxa. Approximately 39% of described fish species of the continent are imperiled. There are 230 vulnerable, 190 threatened, and 280 endangered extant taxa, and 61 taxa presumed extinct or extirpated from nature. Of those that were imperiled in 1989, most (89%) are the same or worse in conservation status; only 6% have improved in status, and 5% were delisted for various reasons. Habitat degradation and nonindigenous species are the main threats to at-risk fishes, many of which are restricted to small ranges. Documenting the diversity and status of rare fishes is a critical step in identifying and implementing appropriate actions necessary for their protection and management. Howard L. Jelks, Frank McCormick, Stephen J. Walsh, Joseph S. Nelson, Noel M. Burkhead, Steven P. Platania, Salvador Contreras-Balderas, Brady A. Porter, Edmundo Díaz-Pardo, Claude B. Renaud, Dean A. Hendrickson, Juan Jacobo Schmitter-Soto, John Lyons, Eric B. Taylor, and Nicholas E. Mandrak, Melvin L. Warren, Jr. Jelks, Walsh, and Burkhead are research McCormick is a biologist with the biologists with the U.S. -

Eastern Sand Darter (Ammocrypta Pellucida) in Canada (Ontario Populations) for the Period 2012 - 2017
Species at Risk Act Recovery Strategy Report Series Report on the Progress of Recovery Strategy Implementation for the Eastern Sand Darter (Ammocrypta pellucida) in Canada (Ontario Populations) for the Period 2012 - 2017 Eastern Sand Darter 2018 c Report on the Progress of Recovery Strategy Implementation for the Eastern Sand Darter 2018 Recommended Citation: Fisheries and Oceans Canada. 2018. Report on the Progress of Recovery Strategy Implementation for the Eastern Sand Darter (Ammocrypta pellucida) in Canada (Ontario Populations) for the Period 2012 – 2017. Species at Risk Act Recovery Strategy Report Series. Fisheries and Oceans Canada, Ottawa. v + 33 p. For copies of the progress report, or for additional information on species at risk, including COSEWIC Status Reports, recovery strategies, residence descriptions, action plans, and other related recovery documents, please visit the Species at Risk Public Registry. Cover illustration: Alan Dextrase, Ontario Ministry of Natural Resources and Forestry Également disponible en français sous le titre « Rapport sur les progrès de la mise en œuvre du programme de rétablissement du dard de sable (Ammocrypta pellucida) au Canada, populations de l’Ontario pour la période 2012-2017» © Her Majesty the Queen in Right of Canada, represented by the Minister of Fisheries and Oceans Canada, 2018. All rights reserved. ISBN 978-0-660-24756-4 Catalogue no. En3-4/122-1-2018E-PDF Content (excluding the cover illustration) may be used without permission, with appropriate credit to the source. i Report on the Progress of Recovery Strategy Implementation for the Eastern Sand Darter 2018 Preface The federal, provincial, and territorial government signatories under the 3Accord for the Protection of Species at Risk (1996) agreed to establish complementary legislation and programs that provide for effective protection of species at risk throughout Canada. -
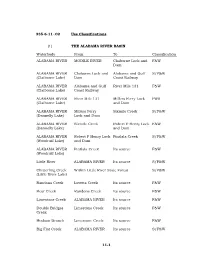
11-1 335-6-11-.02 Use Classifications. (1) the ALABAMA RIVER BASIN Waterbody from to Classification ALABAMA RIVER MOBILE RIVER C
335-6-11-.02 Use Classifications. (1) THE ALABAMA RIVER BASIN Waterbody From To Classification ALABAMA RIVER MOBILE RIVER Claiborne Lock and F&W Dam ALABAMA RIVER Claiborne Lock and Alabama and Gulf S/F&W (Claiborne Lake) Dam Coast Railway ALABAMA RIVER Alabama and Gulf River Mile 131 F&W (Claiborne Lake) Coast Railway ALABAMA RIVER River Mile 131 Millers Ferry Lock PWS (Claiborne Lake) and Dam ALABAMA RIVER Millers Ferry Sixmile Creek S/F&W (Dannelly Lake) Lock and Dam ALABAMA RIVER Sixmile Creek Robert F Henry Lock F&W (Dannelly Lake) and Dam ALABAMA RIVER Robert F Henry Lock Pintlala Creek S/F&W (Woodruff Lake) and Dam ALABAMA RIVER Pintlala Creek Its source F&W (Woodruff Lake) Little River ALABAMA RIVER Its source S/F&W Chitterling Creek Within Little River State Forest S/F&W (Little River Lake) Randons Creek Lovetts Creek Its source F&W Bear Creek Randons Creek Its source F&W Limestone Creek ALABAMA RIVER Its source F&W Double Bridges Limestone Creek Its source F&W Creek Hudson Branch Limestone Creek Its source F&W Big Flat Creek ALABAMA RIVER Its source S/F&W 11-1 Waterbody From To Classification Pursley Creek Claiborne Lake Its source F&W Beaver Creek ALABAMA RIVER Extent of reservoir F&W (Claiborne Lake) Beaver Creek Claiborne Lake Its source F&W Cub Creek Beaver Creek Its source F&W Turkey Creek Beaver Creek Its source F&W Rockwest Creek Claiborne Lake Its source F&W Pine Barren Creek Dannelly Lake Its source S/F&W Chilatchee Creek Dannelly Lake Its source S/F&W Bogue Chitto Creek Dannelly Lake Its source F&W Sand Creek Bogue -

City of Jacksonville/Duval County Environmental Review Record CDBG-DR – Hurricane Matthew Housing Repair Program Contents Scope of Work
City of Jacksonville/Duval County Environmental Review Record CDBG-DR – Hurricane Matthew Housing Repair Program Contents Scope of Work ................................................................................................................................ 1 Broad-Level Tiered Environmental Review ................................................................................... 3 Supporting Documentation ....................................................................................................... 11 Unmet Needs ......................................................................................................................... 11 Project Area ........................................................................................................................... 12 Airport Hazards ..................................................................................................................... 13 Coastal Barrier Resources ..................................................................................................... 17 Flood Insurance ..................................................................................................................... 19 Clean Air................................................................................................................................ 22 Coastal Zone Management .................................................................................................... 26 Endangered Species .............................................................................................................. -

Chapter 335-6-11 Water Use Classifications for Interstate and Intrastate Waters
Environmental Management Chapter 335-6-11 DEPARTMENT OF ENVIRONMENTAL MANAGEMENT WATER DIVISION - WATER QUALITY PROGRAM ADMINISTRATIVE CODE CHAPTER 335-6-11 WATER USE CLASSIFICATIONS FOR INTERSTATE AND INTRASTATE WATERS TABLE OF CONTENTS 335-6-11-.01 The Use Classification System 335-6-11-.02 Use Classifications 335-6-11-.01 The Use Classification System. (1) Use classifications utilized by the State of Alabama are as follows: Outstanding Alabama Water ................... OAW Public Water Supply ......................... PWS Swimming and Other Whole Body Shellfish Harvesting ........................ SH Fish and Wildlife ........................... F&W Limited Warmwater Fishery ................... LWF Agricultural and Industrial Water Supply ................................ A&I (2) Use classifications apply water quality criteria adopted for particular uses based on existing utilization, uses reasonably expected in the future, and those uses not now possible because of correctable pollution but which could be made if the effects of pollution were controlled or eliminated. Of necessity, the assignment of use classifications must take into consideration the physical capability of waters to meet certain uses. (3) Those use classifications presently included in the standards are reviewed informally by the Department's staff as the need arises, and the entire standards package, to include the use classifications, receives a formal review at least once every three years. Efforts currently underway through local 201 planning projects will provide additional technical data on certain waterbodies in the State, information on treatment alternatives, and applicability of various management techniques, which, when available, will hopefully lead to new decisions regarding use classifications. Of particular interest are those segments which are currently classified for any usage which has an associated Supp. -

Low-Head Dams Facilitate Round Goby Neogobius Melanostomus Invasion
Biol Invasions (2018) 20:757–776 https://doi.org/10.1007/s10530-017-1573-3 ORIGINAL PAPER Low-head dams facilitate Round Goby Neogobius melanostomus invasion Dustin Raab . Nicholas E. Mandrak . Anthony Ricciardi Received: 9 July 2017 / Accepted: 23 September 2017 / Published online: 3 October 2017 Ó Springer International Publishing AG 2017 Abstract Round Goby Neogobius melanostomus inclusion of both reservoir-associated abiotic variables invasion of the Grand River (Ontario, Canada) and Round Goby abundance as model terms. To presents an opportunity to assess the role of abiotic determine establishment potential of the uninvaded gradients in mediating the establishment and impact of reach immediately upstream, four environmental nonnative benthic fishes in rivers. In this system, habitat characteristics were used in discriminant sequential low-head dams delineate uninvaded and function analysis (DFA) to predict three potential invaded river reaches and create upstream gradients of outcomes of introduction: non-invaded and either increasing water velocity. We hypothesized that flow lower or higher Round Goby abundance (low and high refugia created by impounded reservoirs above low- invasion status, respectively) than the median number head dams enhance local Round Goby abundance. of Round Goby at invaded sites. Our DFA function Round Goby influence on the native fish community correctly classified non-invaded and high-abundance was determined by variance partitioning, and we used invasion status sites [ 85% of the time, with lower generalized additive models to identify small-bodied (73%) success in classifying low-abundance invasion benthic fish species most likely to be impacted by status sites, and the spatial pattern of our results Round Goby invasion. -

Underwater Observation and Habitat Utilization of Three Rare Darters
University of Tennessee, Knoxville TRACE: Tennessee Research and Creative Exchange Masters Theses Graduate School 5-2010 Underwater observation and habitat utilization of three rare darters (Etheostoma cinereum, Percina burtoni, and Percina williamsi) in the Little River, Blount County, Tennessee Robert Trenton Jett University of Tennessee - Knoxville, [email protected] Follow this and additional works at: https://trace.tennessee.edu/utk_gradthes Part of the Natural Resources and Conservation Commons Recommended Citation Jett, Robert Trenton, "Underwater observation and habitat utilization of three rare darters (Etheostoma cinereum, Percina burtoni, and Percina williamsi) in the Little River, Blount County, Tennessee. " Master's Thesis, University of Tennessee, 2010. https://trace.tennessee.edu/utk_gradthes/636 This Thesis is brought to you for free and open access by the Graduate School at TRACE: Tennessee Research and Creative Exchange. It has been accepted for inclusion in Masters Theses by an authorized administrator of TRACE: Tennessee Research and Creative Exchange. For more information, please contact [email protected]. To the Graduate Council: I am submitting herewith a thesis written by Robert Trenton Jett entitled "Underwater observation and habitat utilization of three rare darters (Etheostoma cinereum, Percina burtoni, and Percina williamsi) in the Little River, Blount County, Tennessee." I have examined the final electronic copy of this thesis for form and content and recommend that it be accepted in partial fulfillment of the equirr ements for the degree of Master of Science, with a major in Wildlife and Fisheries Science. James L. Wilson, Major Professor We have read this thesis and recommend its acceptance: David A. Etnier, Jason G.