A Comparison of the Bering Sea, Gulf of Alaska, and Aleutian Islands Large Marine Ecosystems Through Food Web Modeling
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Research Plan 2 3 A
Arctic Pre-proposal 3.4-Galloway 1 Research Plan 2 3 A. Project Title: Characterizing lipid production hotspots, phenology, and trophic transfer at the 4 algae-herbivore interface in the Chukchi Sea 5 6 B. Category: 3. Oceanography and lower trophic level productivity: Influence of sea ice dynamics and 7 advection on the phenology, magnitude and location of primary and secondary production, match- 8 mismatch, benthic-pelagic coupling, and the influence of winter conditions. 9 10 C. Rationale and justification: 11 The spatial extent of arctic sea ice is declining and earlier seasonal sea ice melt may dramatically 12 affect the magnitude, and spatial and temporal scale of primary production (Kahru et al. 2011, Wassmann 13 2011). In order to predict the consequences of these changes to ecosystems, it is important that we 14 understand the mechanistic links between temporally dynamic ice conditions and the physical factors 15 which govern phytoplankton growth (Popova et al. 2010). The mechanisms that govern productivity of 16 the Chukchi Sea ecosystem are of considerable interest due to dramatically changing temporal and spatial 17 patterns of sea ice coverage and because this area is likely to be the subject of intense fossil fuel 18 exploration in coming decades (Dunton et al. 2014). Tracing the biochemical pathways of basal resources 19 (pelagic and attached micro- and macroalgae) in this system is critical if we are to better understand how 20 the Chukchi Sea ecosystem might be modified in the future by a changing climate and offshore oil and 21 gas exploration and production (McTigue and Dunton 2014). -

Faunal Assemblage Structure on the Patton Seamount (Gulf of Alaska, USA)
Faunal Assemblage Structure on the Patton Seamount (Gulf of Alaska, USA) Gerald R. Hoff and Bradley Stevens Reprinted from the Alaska Fishery Research Bulletin Vol. 11 No. 1, Summer 2005 The Alaska Fisheries Research Bulletin can be found on the World Wide Web at URL: http://www.adfg.state.ak.us/pubs/afrb/afrbhome.php Alaska Fishery Research Bulletin 11(1):27–36. 2005. Copyright © 2005 by the Alaska Department of Fish and Game Faunal Assemblage Structure on the Patton Seamount (Gulf of Alaska, USA) Gerald R. Hoff and Bradley Stevens ABSTRACT: Epibenthic and demersal assemblages of fish and invertebrates on the Patton Seamount in the Gulf of Alaska, U.S.A., were studied in July 1999 using the Deep Sea Research Vehicle Alvin. Faunal associations with depth were described using video analysis of 8 dives from 151 to 3,375 m. A cluster analysis applied to the observations suggests three benthic faunal communities based on depth: 1) a shallow-water community (151–950 m) consisting mainly of rockfishes, flatfishes, sea stars, and attached suspension feeders, 2) a mid-depth community (400–1500 m) also consisting of numerous attached suspension-feeding organisms such as corals, sponges, crinoids, sea anemones, and sea cucumbers and fish such as the sablefishAnoplopoma fimbria and the giant grenadier Albatrossia pectoralis both of which were aggregated over a relatively narrow depth range, and 3) a deep-water community (500–3,375 m) consisting of fewer attached suspension feeders and more highly mobile species such as the Pacific grenadier Coryphaenoides acrolepis, popeye grenadier C. cinereus, Pacific flatnose Antimora microlepis, and large mobile crabs Macroregonia macrochira and Chionoecetes spp. -

The 2014 Golden Gate National Parks Bioblitz - Data Management and the Event Species List Achieving a Quality Dataset from a Large Scale Event
National Park Service U.S. Department of the Interior Natural Resource Stewardship and Science The 2014 Golden Gate National Parks BioBlitz - Data Management and the Event Species List Achieving a Quality Dataset from a Large Scale Event Natural Resource Report NPS/GOGA/NRR—2016/1147 ON THIS PAGE Photograph of BioBlitz participants conducting data entry into iNaturalist. Photograph courtesy of the National Park Service. ON THE COVER Photograph of BioBlitz participants collecting aquatic species data in the Presidio of San Francisco. Photograph courtesy of National Park Service. The 2014 Golden Gate National Parks BioBlitz - Data Management and the Event Species List Achieving a Quality Dataset from a Large Scale Event Natural Resource Report NPS/GOGA/NRR—2016/1147 Elizabeth Edson1, Michelle O’Herron1, Alison Forrestel2, Daniel George3 1Golden Gate Parks Conservancy Building 201 Fort Mason San Francisco, CA 94129 2National Park Service. Golden Gate National Recreation Area Fort Cronkhite, Bldg. 1061 Sausalito, CA 94965 3National Park Service. San Francisco Bay Area Network Inventory & Monitoring Program Manager Fort Cronkhite, Bldg. 1063 Sausalito, CA 94965 March 2016 U.S. Department of the Interior National Park Service Natural Resource Stewardship and Science Fort Collins, Colorado The National Park Service, Natural Resource Stewardship and Science office in Fort Collins, Colorado, publishes a range of reports that address natural resource topics. These reports are of interest and applicability to a broad audience in the National Park Service and others in natural resource management, including scientists, conservation and environmental constituencies, and the public. The Natural Resource Report Series is used to disseminate comprehensive information and analysis about natural resources and related topics concerning lands managed by the National Park Service. -
Schedule Onlinepdf
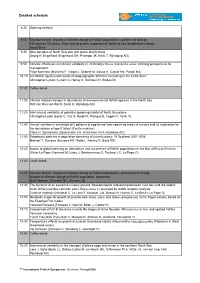
Detailed schedule 8:30 Opening session 9:00 Keynote lecture: Impacts of climate change on flatfish populations - patterns of change 100 days to 100 years: Short and long-term responses of flatfish to sea temperature change David Sims 9:30 Nine decades of North Sea sole and plaice distributions Georg H. Engelhard (Engelhard GH, Pinnegar JK, Kell LT, Rijnsdorp AD) 9:50 Climatic effects on recruitment variability in Platichthys flesus and Solea solea: defining perspectives for management. Filipe Martinho (Martinho F, Viegas I, Dolbeth M, Sousa H, Cabral HN, Pardal MA) 10:10 Are flatfish species with southern biogeographic affinities increasing in the Celtic Sea? Christopher Lynam (Lynam C, Harlay X, Gerritsen H, Stokes D) 10:30 Coffee break 11:00 Climate related changes in abundance of non-commercial flatfish species in the North Sea Ralf van Hal (van Hal R, Smits K, Rijnsdorp AD) 11:20 Inter-annual variability of potential spawning habitat of North Sea plaice Christophe Loots (Loots C, Vaz S, Koubii P, Planque B, Coppin F, Verin Y) 11:40 Annual variation in simulated drift patterns of egg/larvae from spawning areas to nursery and its implication for the abundance of age-0 turbot (Psetta maxima) Claus R. Sparrevohn (Sparrevohn CR, Hinrichsen H-H, Rijnsdorp AD) 12:00 Broadscale patterns in population dynamics of juvenile plaice: W Scotland 2001-2008 Michael T. Burrows (Burrows MT, Robb L, Harvey R, Batty RS) 12:20 Impact of global warming on abundance and occurrence of flatfish populations in the Bay of Biscay (France) Olivier Le Pape (Hermant -

CHECKLIST and BIOGEOGRAPHY of FISHES from GUADALUPE ISLAND, WESTERN MEXICO Héctor Reyes-Bonilla, Arturo Ayala-Bocos, Luis E
ReyeS-BONIllA eT Al: CheCklIST AND BIOgeOgRAphy Of fISheS fROm gUADAlUpe ISlAND CalCOfI Rep., Vol. 51, 2010 CHECKLIST AND BIOGEOGRAPHY OF FISHES FROM GUADALUPE ISLAND, WESTERN MEXICO Héctor REyES-BONILLA, Arturo AyALA-BOCOS, LUIS E. Calderon-AGUILERA SAúL GONzáLEz-Romero, ISRAEL SáNCHEz-ALCántara Centro de Investigación Científica y de Educación Superior de Ensenada AND MARIANA Walther MENDOzA Carretera Tijuana - Ensenada # 3918, zona Playitas, C.P. 22860 Universidad Autónoma de Baja California Sur Ensenada, B.C., México Departamento de Biología Marina Tel: +52 646 1750500, ext. 25257; Fax: +52 646 Apartado postal 19-B, CP 23080 [email protected] La Paz, B.C.S., México. Tel: (612) 123-8800, ext. 4160; Fax: (612) 123-8819 NADIA C. Olivares-BAñUELOS [email protected] Reserva de la Biosfera Isla Guadalupe Comisión Nacional de áreas Naturales Protegidas yULIANA R. BEDOLLA-GUzMáN AND Avenida del Puerto 375, local 30 Arturo RAMíREz-VALDEz Fraccionamiento Playas de Ensenada, C.P. 22880 Universidad Autónoma de Baja California Ensenada, B.C., México Facultad de Ciencias Marinas, Instituto de Investigaciones Oceanológicas Universidad Autónoma de Baja California, Carr. Tijuana-Ensenada km. 107, Apartado postal 453, C.P. 22890 Ensenada, B.C., México ABSTRACT recognized the biological and ecological significance of Guadalupe Island, off Baja California, México, is Guadalupe Island, and declared it a Biosphere Reserve an important fishing area which also harbors high (SEMARNAT 2005). marine biodiversity. Based on field data, literature Guadalupe Island is isolated, far away from the main- reviews, and scientific collection records, we pres- land and has limited logistic facilities to conduct scien- ent a comprehensive checklist of the local fish fauna, tific studies. -

Recent Declines in Warming and Vegetation Greening Trends Over Pan-Arctic Tundra
Remote Sens. 2013, 5, 4229-4254; doi:10.3390/rs5094229 OPEN ACCESS Remote Sensing ISSN 2072-4292 www.mdpi.com/journal/remotesensing Article Recent Declines in Warming and Vegetation Greening Trends over Pan-Arctic Tundra Uma S. Bhatt 1,*, Donald A. Walker 2, Martha K. Raynolds 2, Peter A. Bieniek 1,3, Howard E. Epstein 4, Josefino C. Comiso 5, Jorge E. Pinzon 6, Compton J. Tucker 6 and Igor V. Polyakov 3 1 Geophysical Institute, Department of Atmospheric Sciences, College of Natural Science and Mathematics, University of Alaska Fairbanks, 903 Koyukuk Dr., Fairbanks, AK 99775, USA; E-Mail: [email protected] 2 Institute of Arctic Biology, Department of Biology and Wildlife, College of Natural Science and Mathematics, University of Alaska, Fairbanks, P.O. Box 757000, Fairbanks, AK 99775, USA; E-Mails: [email protected] (D.A.W.); [email protected] (M.K.R.) 3 International Arctic Research Center, Department of Atmospheric Sciences, College of Natural Science and Mathematics, 930 Koyukuk Dr., Fairbanks, AK 99775, USA; E-Mail: [email protected] 4 Department of Environmental Sciences, University of Virginia, 291 McCormick Rd., Charlottesville, VA 22904, USA; E-Mail: [email protected] 5 Cryospheric Sciences Branch, NASA Goddard Space Flight Center, Code 614.1, Greenbelt, MD 20771, USA; E-Mail: [email protected] 6 Biospheric Science Branch, NASA Goddard Space Flight Center, Code 614.1, Greenbelt, MD 20771, USA; E-Mails: [email protected] (J.E.P.); [email protected] (C.J.T.) * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +1-907-474-2662; Fax: +1-907-474-2473. -

Bulletin of the United States Fish Commission
CONTRIBUTIONS FROM THE BIOLOGICAL LABORATORY OF THE U. S. FISH COMMISSION AT WOODS HOLE, MASSACHUSETTS. THE ECHINODERMS OE THE WOODS HOLE REGION. BY HUBERT LYMAN CLARK, Professor of Biology Olivet College .. , F. C. B. 1902—35 545 G’G-HTEMrS. Page. Echinoidea: Page. Introduction 547-550 Key to the species 562 Key to the classes 551 Arbacia punctlilata 563 Asteroidea: Strongylocentrotus drbbachiensis 563 Key to the species 552 Echinaiachnius parma 564 Asterias forbesi 552 Mellita pentapora 565 Asterias vulgaris 553 Holothurioidea: Asterias tenera 554 Key to the species 566 Asterias austera 555 Cucumaria frondosa 566 Cribrella sanguinolenta 555 Cucumaria plulcherrkna 567 Solaster endeca 556 Thyone briareus 567 Ophiuroidea: Thyone scabra 568 Key to the species 558 Thyone unisemita 569 Ophiura brevi&pina 558 Caudina arenata 569 Ophioglypha robusta 558 Trocliostoma oolitieum 570 Ophiopholis aculeata 559 Synapta inhaerens 571 Amphipholi.s squamata 560 Synapta roseola 571 _ Gorgonocephalus agassizii , 561 Bibliography 572-574 546 THE ECHINODERMS OF THE WOODS HOLE REGION. By HUBERT LYMAN CLARK, Professor of Biology , Olivet College. As used in this report, the Woods Hole region includes that part of I he New England coast easily accessible in one-day excursions by steamer from the U. S. Fish Commission station at Woods Hole, Mass. The northern point of Cape Cod is the limit in one direction, and New London, Conn., is the opposite extreme. Seaward the region would naturally extend to about the 100- fathom line, but for the purposes of this report the 50-fathom line has been taken as the limit, the reason for this being that as the Gulf Stream is approached we meet with an echinodcrm fauna so totally different from that along shore that the two have little in common. -

Ascidiacea: Polyclinidae) from the Strait of Gibraltar A
Research Article Mediterranean Marine Science Indexed in WoS (Web of Science, ISI Thomson) and SCOPUS The journal is available on line at http://www.medit-mar-sc.net DOI: http://dx.doi.org/10.12681/mms.1940 A striking morphotype of Aplidium proliferum (Milne Edwards, 1841) (Ascidiacea: Polyclinidae) from the Strait of Gibraltar A. A. RAMOS-ESPLA1 and O. OCAÑA2 1 Marine Research Centre (CIMAR), University of Alicante, 03080 Alicante, Spain 2 Museo del Mar de Ceuta, Muelle España, s/n, 51001 Ceuta, Spain Corresponding author: [email protected] Handling Editor: Xavier Turon Received: 15 October 2016; Accepted: 16 December 2016; Published on line: 31 March 2017 Abstract An unusual colonial ascidian with 1-2m in length, belonging to the genus Aplidium (Ascidiacea: Polyclinidae), has been sampled from the Strait of Gibraltar (Ras Leona, Morocco). The characteristics of the colony, zooids and larvae point us to A. pro- liferum. The species seems common in the NE Atlantic from the Shetland Islands to Mediterranean Sea, but the length of colonies found in the Strait this area have not previously been observed. Probably, it is one of the longest ascidians reported worldwide. Keywords: Keywords: Ascidiacea, Polyclinidae, Aplidium, colony size, Strait of Gibraltar, Mediterranean Sea. Introduction meyer, 1924: 211. Harant & Vernières, 1933: 88. Thomp- son, 1934: 36; pl. 35, pl. 35, figs. 6-7; chart 35. Pérès, Aplidium is the genus woth the greatest number of 1956: 290, 295. known species of the class Ascidiacea, with 280 spp. Aplidium proliferum: Berrill, 1950: 102; fig. 29. Kott, (Ascidiacea World Database: www.marinespecies.org/ 1952: 74; fig. -

Preliminary Mass-Balance Food Web Model of the Eastern Chukchi Sea
NOAA Technical Memorandum NMFS-AFSC-262 Preliminary Mass-balance Food Web Model of the Eastern Chukchi Sea by G. A. Whitehouse U.S. DEPARTMENT OF COMMERCE National Oceanic and Atmospheric Administration National Marine Fisheries Service Alaska Fisheries Science Center December 2013 NOAA Technical Memorandum NMFS The National Marine Fisheries Service's Alaska Fisheries Science Center uses the NOAA Technical Memorandum series to issue informal scientific and technical publications when complete formal review and editorial processing are not appropriate or feasible. Documents within this series reflect sound professional work and may be referenced in the formal scientific and technical literature. The NMFS-AFSC Technical Memorandum series of the Alaska Fisheries Science Center continues the NMFS-F/NWC series established in 1970 by the Northwest Fisheries Center. The NMFS-NWFSC series is currently used by the Northwest Fisheries Science Center. This document should be cited as follows: Whitehouse, G. A. 2013. A preliminary mass-balance food web model of the eastern Chukchi Sea. U.S. Dep. Commer., NOAA Tech. Memo. NMFS-AFSC-262, 162 p. Reference in this document to trade names does not imply endorsement by the National Marine Fisheries Service, NOAA. NOAA Technical Memorandum NMFS-AFSC-262 Preliminary Mass-balance Food Web Model of the Eastern Chukchi Sea by G. A. Whitehouse1,2 1Alaska Fisheries Science Center 7600 Sand Point Way N.E. Seattle WA 98115 2Joint Institute for the Study of the Atmosphere and Ocean University of Washington Box 354925 Seattle WA 98195 www.afsc.noaa.gov U.S. DEPARTMENT OF COMMERCE Penny. S. Pritzker, Secretary National Oceanic and Atmospheric Administration Kathryn D. -

LAYSAN ALBATROSS Phoebastria Immutabilis
Alaska Seabird Information Series LAYSAN ALBATROSS Phoebastria immutabilis Conservation Status ALASKA: High N. AMERICAN: High Concern GLOBAL: Vulnerable Breed Eggs Incubation Fledge Nest Feeding Behavior Diet Nov-July 1 ~ 65 d 165 d ground scrape surface dip fish, squid, fish eggs and waste Life History and Distribution Laysan Albatrosses (Phoebastria immutabilis) breed primarily in the Hawaiian Islands, but they inhabit Alaskan waters during the summer months to feed. They are the 6 most abundant of the three albatross species that visit 200 en Alaska. l The albatross has been described as the “true nomad ff Pok e of the oceans.” Once fledged, it remains at sea for three to J ht ig five years before returning to the island where it was born. r When birds are eight or nine years old they begin to breed. y The breeding season is November to July and the rest of Cop the year, the birds remain at sea. Strong, effortless flight is commonly seen in the southern Bering Sea, Aleutian the key to being able to spend so much time in the air. The Islands, and the northwestern Gulf of Alaska. albatross takes advantage of air currents just above the Nonbreeders may remain in Alaska throughout the year ocean's waves to soar in perpetual fluid motion. It may not and breeding birds are known to travel from Hawaii to flap its wings for hours, or even for days. The aerial Alaska in search of food for their young. Albatrosses master never touches land outside the breeding season, but have the ability to concentrate the food they catch and it does rest on the water to feed and sleep. -

Updated Checklist of Marine Fishes (Chordata: Craniata) from Portugal and the Proposed Extension of the Portuguese Continental Shelf
European Journal of Taxonomy 73: 1-73 ISSN 2118-9773 http://dx.doi.org/10.5852/ejt.2014.73 www.europeanjournaloftaxonomy.eu 2014 · Carneiro M. et al. This work is licensed under a Creative Commons Attribution 3.0 License. Monograph urn:lsid:zoobank.org:pub:9A5F217D-8E7B-448A-9CAB-2CCC9CC6F857 Updated checklist of marine fishes (Chordata: Craniata) from Portugal and the proposed extension of the Portuguese continental shelf Miguel CARNEIRO1,5, Rogélia MARTINS2,6, Monica LANDI*,3,7 & Filipe O. COSTA4,8 1,2 DIV-RP (Modelling and Management Fishery Resources Division), Instituto Português do Mar e da Atmosfera, Av. Brasilia 1449-006 Lisboa, Portugal. E-mail: [email protected], [email protected] 3,4 CBMA (Centre of Molecular and Environmental Biology), Department of Biology, University of Minho, Campus de Gualtar, 4710-057 Braga, Portugal. E-mail: [email protected], [email protected] * corresponding author: [email protected] 5 urn:lsid:zoobank.org:author:90A98A50-327E-4648-9DCE-75709C7A2472 6 urn:lsid:zoobank.org:author:1EB6DE00-9E91-407C-B7C4-34F31F29FD88 7 urn:lsid:zoobank.org:author:6D3AC760-77F2-4CFA-B5C7-665CB07F4CEB 8 urn:lsid:zoobank.org:author:48E53CF3-71C8-403C-BECD-10B20B3C15B4 Abstract. The study of the Portuguese marine ichthyofauna has a long historical tradition, rooted back in the 18th Century. Here we present an annotated checklist of the marine fishes from Portuguese waters, including the area encompassed by the proposed extension of the Portuguese continental shelf and the Economic Exclusive Zone (EEZ). The list is based on historical literature records and taxon occurrence data obtained from natural history collections, together with new revisions and occurrences. -

Find Alaska Info!
Find Alaska Info! Dear Student: Thank you for writing to request information about Alaska. This fl yer contains some interesting information about our great state. Alaska became the 49th state in 1959, right before Hawaii became the 50th state that same year. Many of Alaska’s 722,200 people live in modern cities, and many live in small remote villages where their families have lived for thousands of years. The population of Anchorage, Alaska’s largest city, is 296,000. Juneau (population 32,300) is the State Capital.. We also have a website where you can learn more about Alaska’s history, cultures, geography, animals, and more: http://alaska.gov/kids/ Here are other helpful websites: • For information about visiting Alaska, visit www.travelalaska.com • You can also visit the Alaska State Library on-line at www.lam.alaska.gov • Looking for wildlife info? Go to http://www.wildlife.alaska.gov • Want to know more about Alaska’s Forest and Park Lands? visit www.alaskacenters.gov Did you know? If you place Alaska, with all of its islands, on top of the “continental” United States, it spans from the Great Lakes to Texas, and from Florida to California. At 591,000 square miles, Alaska is larger than Texas, California, and Montana combined. The coastline of Alaska is longer than the coastline of the continental United States. Of Alaska’s 3 million lakes, the largest (Lake Iliamna) is the size of Connecticut. Alaska’s mainland is only 51 miles away from Russia. Alaska has 17 of the 20 highest mountains in North America (Denali is the highest at 20,320 feet).