Canonical Wnt Mechanisms in Neural Crest Induction
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Stewardship Report
Stewardship Report Presented to: The H Foundation December 2015 Our Appreciation Dear Friends of The H Foundation: The Robert H. Lurie Comprehensive Cancer Center of Northwestern University is deeply grateful for The H Foundation’s extraordinary support over the past 15 years. Since 2002, The H Foundation has raised and donated more than $3 million to advance research at the Lurie Cancer Center. In turn, the Lurie Cancer Center has been able to leverage these generous philanthropic funds into more than $50 million in private and government grants for cancer research projects that bridge basic science and clinical care. I am delighted to share this report featuring the faculty members who have received Bridge Awards, Pilot Awards, and other support during 2014-2015. Your giving continues to impact the investigations of our highly dedicated scientists as they work together to find a cure for cancer. Over the years, The H Foundation’s partnership has helped us to stimulate and enable creative, novel research that has led to fundamental new discoveries, accelerating progress towards achieving new diagnostics and treatments for cancer. At the Lurie Cancer Center, we are developing programs that bridge basic science and clinical care and that will establish Chicago as a global leader in the delivery of personalized cancer treatment. Established in 1974, the Lurie Cancer Center is recognized as one of just 45 National Cancer Institute (NCI)-designated “comprehensive” cancer centers in the country for its dedication to the highest standards of cancer research, patient care, education, and community outreach. Our center is a founding member of the National Comprehensive Cancer Network and the Big Ten Cancer Research Consortium. -

2020 Program Book
PROGRAM BOOK Note that TAGC was cancelled and held online with a different schedule and program. This document serves as a record of the original program designed for the in-person meeting. April 22–26, 2020 Gaylord National Resort & Convention Center Metro Washington, DC TABLE OF CONTENTS About the GSA ........................................................................................................................................................ 3 Conference Organizers ...........................................................................................................................................4 General Information ...............................................................................................................................................7 Mobile App ....................................................................................................................................................7 Registration, Badges, and Pre-ordered T-shirts .............................................................................................7 Oral Presenters: Speaker Ready Room - Camellia 4.......................................................................................7 Poster Sessions and Exhibits - Prince George’s Exhibition Hall ......................................................................7 GSA Central - Booth 520 ................................................................................................................................8 Internet Access ..............................................................................................................................................8 -

The LIM Adaptor Protein LMO4 Is an Essential Regulator of Neural Crest Development
Developmental Biology 361 (2012) 313–325 Contents lists available at SciVerse ScienceDirect Developmental Biology journal homepage: www.elsevier.com/developmentalbiology The LIM adaptor protein LMO4 is an essential regulator of neural crest development Stacy D. Ochoa, Sally Salvador, Carole LaBonne ⁎ Dept. of Molecular Biosciences; and Robert H. Lurie Comprehensive Cancer Center, Northwestern University, Evanston, IL 60208, USA article info abstract Article history: The neural crest (NC) is a population of multipotent stem cell-like progenitors that arise at the neural plate Received for publication 3 August 2011 border in vertebrates and migrate extensively before giving rise to diverse derivatives. A number of compo- Revised 18 October 2011 nents of the neural crest gene regulatory network (NC-GRN) are used reiteratively to control multiple steps Accepted 21 October 2011 in the development of these cells. It is therefore important to understand the mechanisms that control the Available online 18 November 2011 distinct function of reiteratively used factors in different cellular contexts, and an important strategy for doing so is to identify and characterize the regulatory factors they interact with. Here we report that the Keywords: Xenopus LIM adaptor protein, LMO4, is a Slug/Snail interacting protein that is essential for NC development. LMO4 Neural crest is expressed in NC forming regions of the embryo, as well as in the central nervous system and the cranial LIM placodes. LMO4 is necessary for normal NC development as morpholino-mediated knockdown of this factor Snail leads to loss of NC precursor formation at the neural plate border. Misexpression of LMO4 leads to ectopic ex- Slug pression of some neural crest markers, but a reduction in the expression of others. -

FGF Mediated MAPK and PI3K/Akt Signals Make Distinct Contributions to Pluripotency and the Establishment of Neural Crest Lauren Geary1, Carole Labonne1,2*
RESEARCH ARTICLE FGF mediated MAPK and PI3K/Akt Signals make distinct contributions to pluripotency and the establishment of Neural Crest Lauren Geary1, Carole LaBonne1,2* 1Department of Molecular Biosciences, Northwestern University, Evanston, United States; 2Robert H Lurie Comprehensive Cancer Center, Northwestern University, Evanston, United States Abstract Early vertebrate embryos possess cells with the potential to generate all embryonic cell types. While this pluripotency is progressively lost as cells become lineage restricted, Neural Crest cells retain broad developmental potential. Here, we provide novel insights into signals essential for both pluripotency and neural crest formation in Xenopus. We show that FGF signaling controls a subset of genes expressed by pluripotent blastula cells, and find a striking switch in the signaling cascades activated by FGF signaling as cells lose pluripotency and commence lineage restriction. Pluripotent cells display and require Map Kinase signaling, whereas PI3 Kinase/Akt signals increase as developmental potential is restricted, and are required for transit to certain lineage restricted states. Importantly, retaining a high Map Kinase/low Akt signaling profile is essential for establishing Neural Crest stem cells. These findings shed important light on the signal- mediated control of pluripotency and the molecular mechanisms governing genesis of Neural Crest. DOI: https://doi.org/10.7554/eLife.33845.001 *For correspondence: [email protected] Competing interests: The Introduction authors declare that no The evolutionary transition from simple chordate body plans to complex vertebrate body plans was competing interests exist. driven by the acquisition of the Neural Crest, a unique stem cell population with broad, multi-germ Funding: See page 17 layer developmental potential (Le Douarin and Kalcheim, 1999; Hall, 2000; Bronner and LeDouarin, 2012; Prasad et al., 2012). -
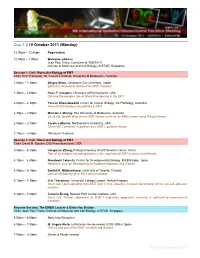
10 October 2011 (Monday)
Day 1 | 10 October 2011 (Monday) 12.00pm – 12.45pm Registration 12.45pm – 1.00pm Welcome address Jean Paul Thiery, Convenor of TEMTIA-V Institute of Molecular and Cell Biology, A*STAR, Singapore Session 1: Cell / Molecular Biology of EMT Chair: Erik Thompson, St. Vincent’s Institute, University of Melbourne, Australia 1.00pm – 1.30pm Shigeo Ohno, Yokohama City University, Japan Epithelial cell polarity and the Par-aPKC complex 1.30pm – 2.00pm Russ P. Carstens, University of Pennsylvania, USA Defining the complex role of alternative splicing in the EMT 2.00pm – 2.30pm Yeesim Khew-Goodall, Centre for Cancer Biology, SA Pathology, Australia The miR-200 family of microRNAs in EMT 2.30pm – 2.50pm Michael J. Murray, The University of Melbourne, Australia Short talk: Identification of new EMT factors in the fly: an RNAi screen using Wing Eversion 2.50pm – 3.10pm Carole LaBonne, Northwestern University, USA Short talk: Coordinate regulation core EMT regulatory factors 3.10pm – 3.40pm Afternoon Teabreak Session 2: Cell / Molecular Biology of EMT Chair: David M. Epstein, OSI Pharmaceuticals, USA 3.40pm – 4.10pm Hongquan Zhang, Peking University Health Science Center, China Role of an integrin-interacting protein in the regulation of EMT in cancer and fibrosis 4.10pm – 4.40pm Masatoshi Takeichi, Center for Developmental Biology, RIKEN Kobe, Japan Adherens Junction Remodeling for Epithelial Migration and Closure 4.40pm – 5.10pm Senthil K. Muthuswamy, University of Toronto, Canada Loss of cell polarity gene Par3 and metastasis 5.10pm – 5.30pm Eric -

Program Book
The Genetics Society of America Conferences 15th International Xenopus Conference August 24-28, 2014 • Pacific Grove, CA PROGRAM GUIDE LEGEND Information/Guest Check-In Disabled Parking E EV Charging Station V Beverage Vending Machine N S Ice Machine Julia Morgan Historic Building W Roadway Pedestrian Pathway Outdoor Group Activity Area Program and Abstracts Meeting Organizers Carole LaBonne, Northwestern University John Wallingford, University of Texas at Austin Organizing Committee: Julie Baker, Stanford Univ Chris Field, Harvard Medical School Carmen Domingo, San Francisco State Univ Anna Philpott, Univ of Cambridge 9650 Rockville Pike, Bethesda, Maryland 20814-3998 Telephone: (301) 634-7300 • Fax: (301) 634-7079 E-mail: [email protected] • Web site: genetics-gsa.org Thank You to the Following Companies for their Generous Support Platinum Sponsor Gold Sponsors Additional Support Provided by: Carl Zeiss Microscopy, LLC Monterey Convention & Gene Tools, LLC Visitors Bureau Leica Microsystems Xenopus Express 2 Table of Contents General Information ........................................................................................................................... 5 Schedule of Events ............................................................................................................................. 6 Exhibitors ........................................................................................................................................... 8 Opening Session and Plenary/Platform Sessions ............................................................................ -

SOCIETY for DEVELOPMENTAL BIOLOGY 70TH ANNUAL MEETING July 21 – 25, 2011 Hyatt Regency Riverwalk, Chicago, IL
SOCIETY FOR DEVELOPMENTAL BIOLOGY 70TH ANNUAL MEETING July 21 – 25, 2011 Hyatt Regency Riverwalk, Chicago, IL Program Committee: Alexandra Joyner (Chair, SDB President), Mary Baylies, Jeremy Nance, James Umen and Debbie Yelon Local Committee: Carole LaBonne, Peter Okkema and Vicky Prince Program Abstract Number in bold italics Presenting Author Name in bold Session room in italics Wednesday, July 20 02:00 PM 09:00 PM 2nd SDB Faculty Re-boot Camp Truffles Organizer: Yolanda Cruz, Oberlin Thursday, July 21 08:00 AM 01:00 PM 2nd SDB Faculty Re-boot Camp (continuation) Truffles 08:30 AM 05:00 PM Satellite Symposium 1 (not organized by SDB) Regency C Visualizing Complex Cell Dynamics in the Embryo Organizers: Kat Hadjantonakis, MSKCC, Dan Turnbull, NYU and Paul Kulesa, Stowers 08:30 AM 05:00 PM Satellite Symposium 2 (not organized by SDB) Regency D Translating Pancreatic Development to Treat Diabetes Organizers: Matthias Hebrok, UCSF, Vicky Prince, U Chicago, Chris Rhodes, U Chicago and Lori Sussel, Columbia 01:00 PM 06:00 PM Meeting Registration Regency Main Desk 01:00 PM 06:00 PM Exhibits Set-up Riverside Center West 03:00 PM 05:00 PM Professional Development and Education Committee Meeting Atlanta 03:00 PM 06:00 PM Poster Session I Set-up Riverside Center West Poster Session I themes: Education – Cell Signaling – Intracellular Signaling Pathways – Morphogenesis – Organogenesis Please see poster assignment in the end of the Meeting Program 06:00 PM 08:00 PM Presidential Symposium Regency Ballroom 06:00 PM 06:05 PM Welcome - Alexandra Joyner, SDB President, MSKCC 06:05 PM 06:40 PM Hormonal control of plant growth in response to changes in the environment. -

Xenopus Community White Paper 2009
2011 Xenopus White Paper 1 Xenopus Community White Paper 2011 Contents: Executive Summary: Page 2 Introduction: Page 5 Immediate Needs of the Xenopus Community: Generation of the Xenopus ORFeome Page 6 Improvement of the Xenopus genome sequence Page 8 Essential Resources for Xenopus research: Improvement of long-range contiguity in the Xenopus laevis genome Page 10 Improvement of Xenopus antibody resources Page 12 Loss of function: Zinc Finger Nucleases/TILLING Page 14 Loss of function: Small inhibitory hairpin RNAs Page 16 Novel loss of function/knockdown/knockout technologies Page 18 Intergenic annotation of the Xenopus genome Page 18 Improvement of X. tropicalis genome – long range contiguity Page 20 Additions and improvements to Xenbase: The Xenopus Model Organism Database Page 21 Frogbook: A comprehensive resource for methods in Xenopus biology Page 21 Summaries of contributions by Xenopus research to the missions of the National Institutes of Health: Institute: Authors Page: NIGMS Wallingford, De Robertis, Gautier, and Zheng 23 NCI LaBonne and Gautier 28 NEI Vetter and Moore 31 NHLBI Krieg 34 NHGRI Loots 36 NIAID Robert 39 NIAAA Harris 43 NIBIB Davidson 44 NICHD Wylie and Harland 46 NIDCD Collazo 50 NIDCR Liu and Sive 52 NIDDK Wessely 55 NLM Vize 58 NIEHS Cimprich 59 NIMH Levin and Klein 61 NINDS Kelley 65 Appendix 1 – Contributors to the Xenopus Community White Paper 2011 Page 69 Appendix 2 – Signatories to the Xenopus Community White Paper 2011 Page 70 2011 Xenopus White Paper 2 Executive Summary Xenopus: An essential vertebrate model system for biomedical research: Model animals are crucial to advancing biomedical research. -

Neural Induction in Xenopus Requires Inhibition of Wnt-Β-Catenin Signaling ⁎ Elizabeth Heeg-Truesdell A, Carole Labonne A,B
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Developmental Biology 298 (2006) 71–86 www.elsevier.com/locate/ydbio Neural induction in Xenopus requires inhibition of Wnt-β-catenin signaling ⁎ Elizabeth Heeg-Truesdell a, Carole LaBonne a,b, a Department of Biochemistry, Molecular Biology and Cell Biology, Northwestern University, Evanston, Il 60208, USA b Robert H. Lurie Comprehensive Cancer Center, Northwestern University, Evanston, Il 60208, USA Received for publication 27 February 2006; revised 5 June 2006; accepted 6 June 2006 Available online 14 June 2006 Abstract Canonical Wnt signals have been implicated in multiple events during early embryogenesis, including primary axis formation, neural crest induction, and A–P patterning of the neural plate. The mechanisms by which Wnt signals can direct distinct fates in cell types that are closely linked both temporally and spatially remains poorly understood. However, recent work has suggested that the downstream transcriptional mediators of this pathway, Lef/Tcf family DNA binding proteins, may confer distinct outcomes on these signals in some cellular contexts. In this study, we first examined whether inhibitory mutants of XTcf3 and XLef1 might block distinct Wnt-dependent signaling events during the diversification of cell fates in the early embryonic ectoderm. We found that a Wnt-unresponsive mutant of XTcf3 potently blocks neural crest formation, whereas an analogous mutant of XLef1 does not, and that the difference in activity mapped to the C-terminus of the proteins. Significantly, the inhibitory XTcf3 mutant also blocked expression of markers of anterior-most cell types, including cement gland and sensory placodes, indicating that Wnt signals are required for rostral as well as caudal ectodermal fates. -

SDB Regional Meeting Program with Abstracts
Society for Developmental Biology Midwest Regional Meeting Case Western Reserve University Cleveland, OH September 14-16, 2018 CWRU Campus Map Schedule of Events All events will take place in the Tinkham Veale Center unless otherwise noted with (**) Friday, September 14th 11:00 am-7:00 pm Registration Desk Open Location: Foyer 11:00 am-7:00 pm Poster Set Up Location: Ballroom A 12:00 pm-2:00 pm Emerging Technologies in Developmental Biology Location: Room 134 Workshop 2:00 pm-4:00 pm Education Workshop Location: Ballroom B 4:30 pm-4:45 pm Meeting Opening and Announcements Location: Ballroom B 4:45 pm-6:15 pm Session 1: Experimental and Theoretical Models of Location: Ballroom B Pattern Formation Chair: Adriana Dawes 6:15 pm-6:45 pm Coffee Break Location: Foyer 6:55 pm-8:00 pm Keynote Lecture I Location: Ballroom B Speaker: Carole LaBonne 8:30 pm-10:30 pm Poster Presentation and Social Hour Location: Ballroom A Saturday, September 15th 7:30 am-8:30 am Continental Breakfast Location: Foyer 8:30 am-8:35 am Announcements Location: Ballroom B 8:35 am-10:05 am Session 2: Evolution and Development Location: Ballroom B Chair: John Masly 10:05 am-10:35 am Break Location: Foyer 10:35 am-12:05 pm Session 3: Epigenetics: From Development to Aging Location: Ballroom B Chairs: Joseph Ogas 12:05 pm-3:00 pm Open Poster Viewing 12:30 pm-2:45 pm Emerging Technologies in Developmental Biology Location: Ballroom C Workshop 3:00 pm-4:30 pm Session 4: Organogenesis and Morphogenesis Location: Ballroom B Chair: Michiko Watanabe 4:30 pm-6:30 pm Poster Presentation -

Final Program
Society for Developmental Biology 68th Annual Meeting July 23 – 27, 2009 Hyatt Regency, San Francisco, CA Organizing Committee: Marianne Bronner-Fraser (Chair, SDB President), Claude Desplan, Robb Krumlauf and Andrea Streit Local Organizers: Laura Burrus (SFSU) and Richard Schneider (UCSF) Abstract program numbers are in italics PROGRAM Wednesday, July 22 1 pm – 9 pm First SDB Faculty Re-Boot Camp Marina 1 Chair: Karen L. Bennett. Univ Missouri-Columbia, SDB Professional Development & Education Committee Thursday, July 23 8 am – 1 pm First SDB Faculty Re-Boot Camp (continuation) Marina 1 Chair: Karen L. Bennett. Univ Missouri-Columbia, SDB Professional Development & Education Committtee 8 am – 5 pm Satellite Symposia (not organized by SDB) Symposium I Neural Crest and Ectodermal Placodes Seacliff Co-Chairs: Paul Trainor, Andrea Streit and Robb Krumlauf. Stowers Institute for Medical Research, Kansas City, MO; Kings College London, UK Symposium II Plant Development in a Changing World Bayview Co-Chairs: Dominique Bergmann, Andrew Groover and Chelsea Specht. Stanford University, CA; USDA Forest Service and UC Davis, CA; UC Berkeley, CA 1 – 5 pm SDB Committees Meetings TBA 2 – 6 pm Annual Meeting Registration Grand Ballroom Foyer 3 – 6 pm Exhibits and Poster Session I – set up Pacific Concourse 6 – 8 pm Presidential Symposium Grand Ballroom 6:00 Welcome and introduction Marianne Bronner-Fraser, CALTECH (SDB President) 2 6:15 From Genome to Phenotype: Modeling the Interaction of Physical and Chemical Signals in Plant Meristems. Elliot M. Meyerowitz. Division of Biology 156-29, California Institute of Technology, Pasadena, CA, USA. 6:50 Gastrulation: from cells to embryo Claudio Stern, University College London, UK. -

Society for Developmental Biology
Society for Developmental MidwestBiology Regional Meeting Case Western Reserve University Cleveland, OH September 16-18, 2019 CWRU Campus Map Formatted: Font color: Text 1 C Formatted: Centered Schedule of Events All events will take place in the Tinkham Veale Center Monday, September 16th 12:00 pm-7:00 pm Registration Desk Open Location: Foyer 12:00 pm-5:00 pm Poster Set Up Location: Ballroom A 1:30 pm-3:30 pm Education Workshop: Scientific Writing 101. Fellowships, grants, and papers! Oh my! Location: Ballroom C 3:30 pm-3:35 pm Meeting Opening and Announcements Location: Ballroom B 3:35 pm-5:05 pm Session 1: Experimental and Theoretical Models of Location: Ballroom B Pattern Formation Chair: Anna Dobritsa 5:05pm Coffee Break Location: Foyer 5:05 pm- 6:05 pm Poster Presentations (even numbers) Location: Ballroom A 6:05 pm- 7:05 pm Poster Presentations (odd numbers) Location: Ballroom A 7:10 pm– 8:20 pm Keynote Lecture I Location: Ballroom B Speaker: Tatjana Piotrowski 8:30 pm-10:30 pm Welcome reception and Social Hour Location: Foyer Tuesday, September 17th 7:30 am-8:30 am Continental Breakfast Location: Foyer 8:30 am-8:35 am Announcements Location: Ballroom B 8:35 am-10:05 am Session 2: Evolution and Development Location: Ballroom B Chair: Cora MacAlister 10:05 am-10:35 am Break Location: Foyer 10:35 am-12:05 pm Session 3: Epigenetics: From Development to Aging Location: Ballroom B Chairs: Shelby Blythe 12:05 pm-3:00 pm Open Poster Viewing 12:10 pm-2:40 pm Applications of Single Cell Analysis in Developmental Biology Workshop Location: