What Genomic Data Can Reveal About Eco-Evolutionary Dynamics
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ecological and Evolutionary Dynamics of Interconnectedness and Modularity
Ecological and evolutionary dynamics of interconnectedness and modularity Jan M. Nordbottena,b, Simon A. Levinc, Eörs Szathmáryd,e,f, and Nils C. Stensethg,1 aDepartment of Mathematics, University of Bergen, N-5020 Bergen, Norway; bDepartment of Civil and Environmental Engineering, Princeton University, Princeton, NJ 08544; cDepartment of Ecology and Evolutionary Biology, Princeton University, Princeton, NJ 08544; dParmenides Center for the Conceptual Foundations of Science, D-82049 Pullach, Germany; eMTA-ELTE (Hungarian Academy of Sciences—Eötvös University), Theoretical Biology and Evolutionary Ecology Research Group, Department of Plant Systematics, Ecology and Theoretical Biology, Eötvös University, Budapest, H-1117 Hungary; fEvolutionary Systems Research Group, MTA (Hungarian Academy of Sciences) Ecological Research Centre, Tihany, H-8237 Hungary; and gCentre for Ecological and Evolutionary Synthesis (CEES), Department of Biosciences, University of Oslo, N-0316 Oslo, Norway Contributed by Nils C. Stenseth, December 1, 2017 (sent for review September 12, 2017; reviewed by David C. Krakauer and Günter P. Wagner) In this contribution, we develop a theoretical framework for linking refinement of this inquiry is to look for compartmentalization (i.e., microprocesses (i.e., population dynamics and evolution through modularity) in ecosystems. In food webs, for example, compartments natural selection) with macrophenomena (such as interconnectedness (which are subgroups of taxa with many strong interactions in each and modularity within an -

Martin A. Nowak
EVOLUTIONARY DYNAMICS EXPLORING THE EQUATIONS OF LIFE MARTIN A. NOWAK THE BELKNAP PRESS OF HARVARD UNIVERSITY PRESS CAMBRIDGE, MASSACHUSETTS, AND LONDON, ENGLAND 2006 Copyright © 2006 by the President and Fellows of Harvard College All rights reserved Printed in Canada Library of Congress Cataloging-in-Publication Data Nowak, M. A. (Martin A.) Evolutionary dynamics : exploring the equations of life / Martin A. Nowak. p. cm. Includes bibliographical references and index. ISBN-13: 978-0-674-02338-3 (alk. paper) ISBN-10: 0-674-02338-2 (alk. paper) 1. Evolution (Biology)—Mathematical models. I. Title. QH371.3.M37N69 2006 576.8015118—dc22 2006042693 Designed by Gwen Nefsky Frankfeldt CONTENTS Preface ix 1 Introduction 1 2 What Evolution Is 9 3 Fitness Landscapes and Sequence Spaces 27 4 Evolutionary Games 45 5 Prisoners of the Dilemma 71 6 Finite Populations 93 7 Games in Finite Populations 107 8 Evolutionary Graph Theory 123 9 Spatial Games 145 10 HIV Infection 167 11 Evolution of Virulence 189 12 Evolutionary Dynamics of Cancer 209 13 Language Evolution 249 14 Conclusion 287 Further Reading 295 References 311 Index 349 PREFACE Evolutionary Dynamics presents those mathematical principles according to which life has evolved and continues to evolve. Since the 1950s biology, and with it the study of evolution, has grown enormously, driven by the quest to understand the world we live in and the stuff we are made of. Evolution is the one theory that transcends all of biology. Any observation of a living system must ultimately be interpreted in the context of its evolution. Because of the tremendous advances over the last half century, evolution has become a dis- cipline that is based on precise mathematical foundations. -

Evolutionary Dynamics on Any Population Structure
Evolutionary dynamics on any population structure Benjamin Allen1,2,3, Gabor Lippner4, Yu-Ting Chen5, Babak Fotouhi1,6, Naghmeh Momeni1,7, Shing-Tung Yau3,8, and Martin A. Nowak1,8,9 1Program for Evolutionary Dynamics, Harvard University, Cambridge, MA, USA 2Department of Mathematics, Emmanuel College, Boston, MA, USA 3Center for Mathematical Sciences and Applications, Harvard University, Cambridge, MA, USA 4Department of Mathematics, Northeastern University, Boston, MA, USA 5Department of Mathematics, University of Tennessee, Knoxville, TN, USA 6Institute for Quantitative Social Sciences, Harvard University, Cambridge, MA, USA 7Department of Electrical and Computer Engineering, McGill University, Montreal, Canada 8Department of Mathematics, Harvard University, Cambridge, MA, USA 9Department of Organismic and Evolutionary Biology, Harvard University, Cambridge, MA, USA December 23, 2016 Abstract Evolution occurs in populations of reproducing individuals. The structure of a biological population affects which traits evolve [1, 2]. Understanding evolutionary game dynamics in structured populations is difficult. Precise results have been absent for a long time, but have recently emerged for special structures where all individuals have the arXiv:1605.06530v2 [q-bio.PE] 22 Dec 2016 same number of neighbors [3, 4, 5, 6, 7]. But the problem of deter- mining which trait is favored by selection in the natural case where the number of neighbors can vary, has remained open. For arbitrary selection intensity, the problem is in a computational complexity class which suggests there is no efficient algorithm [8]. Whether there exists a simple solution for weak selection was unanswered. Here we provide, surprisingly, a general formula for weak selection that applies to any graph or social network. -

Evolutionary Game Theory: a Renaissance
games Review Evolutionary Game Theory: A Renaissance Jonathan Newton ID Institute of Economic Research, Kyoto University, Kyoto 606-8501, Japan; [email protected] Received: 23 April 2018; Accepted: 15 May 2018; Published: 24 May 2018 Abstract: Economic agents are not always rational or farsighted and can make decisions according to simple behavioral rules that vary according to situation and can be studied using the tools of evolutionary game theory. Furthermore, such behavioral rules are themselves subject to evolutionary forces. Paying particular attention to the work of young researchers, this essay surveys the progress made over the last decade towards understanding these phenomena, and discusses open research topics of importance to economics and the broader social sciences. Keywords: evolution; game theory; dynamics; agency; assortativity; culture; distributed systems JEL Classification: C73 Table of contents 1 Introduction p. 2 Shadow of Nash Equilibrium Renaissance Structure of the Survey · · 2 Agency—Who Makes Decisions? p. 3 Methodology Implications Evolution of Collective Agency Links between Individual & · · · Collective Agency 3 Assortativity—With Whom Does Interaction Occur? p. 10 Assortativity & Preferences Evolution of Assortativity Generalized Matching Conditional · · · Dissociation Network Formation · 4 Evolution of Behavior p. 17 Traits Conventions—Culture in Society Culture in Individuals Culture in Individuals · · · & Society 5 Economic Applications p. 29 Macroeconomics, Market Selection & Finance Industrial Organization · 6 The Evolutionary Nash Program p. 30 Recontracting & Nash Demand Games TU Matching NTU Matching Bargaining Solutions & · · · Coordination Games 7 Behavioral Dynamics p. 36 Reinforcement Learning Imitation Best Experienced Payoff Dynamics Best & Better Response · · · Continuous Strategy Sets Completely Uncoupled · · 8 General Methodology p. 44 Perturbed Dynamics Further Stability Results Further Convergence Results Distributed · · · control Software and Simulations · 9 Empirics p. -

Evolutionary Dynamics on Graphs
letters to nature Received 24 September; accepted 16 November 2004; doi:10.1038/nature03211. often individuals place offspring into adjacent vertices. The 3 1. MacArthur, R. H. & Wilson, E. O. The Theory of Island Biogeography (Princeton Univ. Press, homogeneous population, described by the Moran process ,is Princeton, 1969). the special case of a fully connected graph with evenly weighted 2. Fisher, R. A., Corbet, A. S. & Williams, C. B. The relation between the number of species and the number of individuals in a random sample of an animal population. J. Anim. Ecol. 12, 42–58 (1943). edges. Spatial structures are described by graphs where vertices 3. Preston, F. W. The commonness, and rarity, of species. Ecology 41, 611–627 (1948). are connected with their nearest neighbours. We also explore 4. Brown, J. H. Macroecology (Univ. Chicago Press, Chicago, 1995). evolution on random and scale-free networks5–7. We determine 5. Hubbell, S. P. A unified theory of biogeography and relative species abundance and its application to the fixation probability of mutants, and characterize those tropical rain forests and coral reefs. Coral Reefs 16, S9–S21 (1997). 6. Hubbell, S. P. The Unified Theory of Biodiversity and Biogeography (Princeton Univ. Press, Princeton, graphs for which fixation behaviour is identical to that of a 7 2001). homogeneous population . Furthermore, some graphs act as 7. Caswell, H. Community structure: a neutral model analysis. Ecol. Monogr. 46, 327–354 (1976). suppressors and others as amplifiers of selection. It is even 8. Bell, G. Neutral macroecology. Science 293, 2413–2418 (2001). possible to find graphs that guarantee the fixation of any 9. -

Diversity and Coevolutionary Dynamics in High-Dimensional Phenotype
Diversity and coevolutionary dynamics in high-dimensional phenotype spaces Michael Doebeli∗ & Iaroslav Ispolatov∗∗ ∗ Departments of Zoology and Mathematics, University of British Columbia, 6270 University Boulevard, Vancouver B.C. Canada, V6T 1Z4; [email protected] ∗∗ Departamento de Fisica, Universidad de Santiago de Chile Casilla 302, Correo 2, Santiago, Chile; [email protected] October 15, 2018 Supporting Material: Appendix A arXiv:1701.07861v2 [q-bio.PE] 10 Feb 2017 RH: Diversity and coevolutionary dynamics Corresponding author: Michael Doebeli, Department of Zoology and Department of Math- ematics, University of British Columbia, 6270 University Boulevard, Vancouver B.C. Canada, V6T 1Z4, Email: [email protected]. 1 Abstract We study macroevolutionary dynamics by extending microevolutionary competition models to long time scales. It has been shown that for a general class of competition models, gradual evolu- tionary change in continuous phenotypes (evolutionary dynamics) can be non-stationary and even chaotic when the dimension of the phenotype space in which the evolutionary dynamics unfold is high. It has also been shown that evolutionary diversification can occur along non-equilibrium trajectories in phenotype space. We combine these lines of thinking by studying long-term co- evolutionary dynamics of emerging lineages in multi-dimensional phenotype spaces. We use a statistical approach to investigate the evolutionary dynamics of many different systems. We find: 1) for a given dimension of phenotype space, the coevolutionary dynamics tends to be fast and non-stationary for an intermediate number of coexisting lineages, but tends to stabilize as the evolving communities reach a saturation level of diversity; and 2) the amount of diversity at the saturation level increases rapidly (exponentially) with the dimension of phenotype space. -

Temporal and Spatial Effects Beyond Replicator Dynamics
Evolutionary game theory: Temporal and spatial effects beyond replicator dynamics Carlos P. Roca a,∗, José A. Cuesta a, Angel Sánchez a,b,c a Grupo Interdisciplinar de Sistemas Complejos (GISC), Departamento de Matemáticas, Universidad Carlos III de Madrid, Avenida de la Universidad 30, 28911 Leganés, Madrid, Spain b Instituto de Biocomputación y Física de Sistemas Complejos (BIFI), Universidad de Zaragoza, Corona de Aragón 42, 50009 Zaragoza, Spain c Instituto de Ciencias Matemáticas CSIC-UAM-UC3M-UCM, 28006 Madrid, Spain Abstract Evolutionary game dynamics is one of the most fruitful frameworks for studying evolution in different disciplines, from Biology to Economics. Within this context, the approach of choice for many researchers is the so-called replicator equation, that describes mathematically the idea that those individuals performing better have more offspring and thus their frequency in the population grows. While very many interesting results have been obtained with this equation in the three decades elapsed since it was first proposed, it is important to realize the limits of its applicability. One particularly relevant issue in this respect is that of non-mean- field effects, that may arise from temporal fluctuations or from spatial correlations, both neglected in the replicator equation. This review discusses these temporal and spatial effects focusing on the non-trivial modifications they induce when compared to the outcome of replicator dynamics. Alongside this question, the hypothesis of linearity and its relation to the choice of the rule for strategy update is also analyzed. The discussion is presented in terms of the emergence of cooperation, as one of the current key problems in Biology and in other disciplines. -
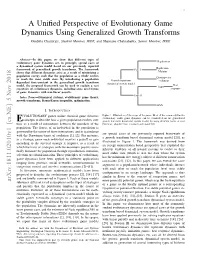
A Unified Perspective of Evolutionary Game Dynamics Using Generalized
1 A Unified Perspective of Evolutionary Game Dynamics Using Generalized Growth Transforms Oindrila Chatterjee, Student Member, IEEE, and Shantanu Chakrabartty, Senior Member, IEEE Abstract—In this paper, we show that different types of evolutionary game dynamics are, in principle, special cases of Replication a dynamical system model based on our previously reported Replicator- framework of generalized growth transforms. The framework Type I shows that different dynamics arise as a result of minimizing a Mutator population energy such that the population as a whole evolves Quasispecies to reach the most stable state. By introducing a population Growth transform evolution dependent time-constant in the generalized growth transform dynamical system model Best model, the proposed framework can be used to explain a vast Logit repertoire of evolutionary dynamics, including some novel forms response of game dynamics with non-linear payoffs. Type II Index Terms—Dynamical systems, evolutionary game theory, growth transforms, Baum-Eagon inequality, optimization. BNN I. INTRODUCTION VOLUTIONARY games utilize classical game theoretic Figure 1. Illustration of the scope of the paper: Most of the commonly known evolutionary stable game dynamics can be obtained from the generalized E concepts to describe how a given population evolves over growth transform dynamical system model, by using different forms of cost time as a result of interactions between the members of the functions, adaptive time constants and manifolds. population. The fitness of an individual in the population is governed by the nature of these interactions, and in accordance are special cases of our previously reported framework of with the Darwinian tenets of evolution [1], [2]. -

Evolutionary Dynamics of Recent Selection on Cognitive Abilities
Evolutionary dynamics of recent selection on cognitive abilities Sara E. Millera, Andrew W. Legana, Michael T. Henshawb, Katherine L. Ostevikc, Kieran Samukc, Floria M. K. Uya,d, and Michael J. Sheehana,1 aDepartment of Neurobiology and Behavior, Cornell University, Ithaca, NY 14853; bDepartment of Biology, Grand Valley State University, Allendale, MI 49401; cDepartment of Biology, Duke University, Durham, NC 27708; and dDepartment of Biology, University of Miami, Coral Gables, FL 33146 Edited by Gene E. Robinson, University of Illinois at Urbana–Champaign, Urbana, IL, and approved December 29, 2019 (received for review November 2, 2019) Cognitive abilities can vary dramatically among species. The relative in cognitive abilities is a heritable quantitative trait (9, 16). Al- importance of social and ecological challenges in shaping cognitive though these approaches have generated many hypotheses for evolution has been the subject of a long-running and recently the selective forces driving the evolution of novel cognitive renewed debate, but little work has sought to understand the abilities, these studies do not provide direct evidence for the selective dynamics underlying the evolution of cognitive abilities. evolutionary dynamics underlying cognitive evolution and, as a Here, we investigate recent selection related to cognition in the result, the process by which cognitive abilities evolve remains an paper wasp Polistes fuscatus—a wasp that has uniquely evolved essentially unexplored question. visual individual recognition abilities. We generate high quality de Analyses of genomic patterns of selection have the potential to novo genome assemblies and population genomic resources for reveal the mode and tempo of cognitive evolution. Prior com- multiple species of paper wasps and use a population genomic parative genomic analyses have identified signatures of positive framework to interrogate the probable mode and tempo of cogni- selection on genes associated with the brain or nervous system in P. -

Evolutionary Cycles of Cooperation and Defection
Evolutionary cycles of cooperation and defection Lorens A. Imhof*†, Drew Fudenberg‡, and Martin A. Nowak§ *Institut fu¨ r Gesellschafts und Wirtschaftswissenschaften, Statistische Abteilung, Universita¨ t Bonn, D-53113 Bonn, Germany; and ‡Department of Economics and §Program for Evolutionary Dynamics, Department of Mathematics, and Department of Organismic and Evolutionary Biology, Harvard University, Cambridge MA 02138 Edited by Robert M. May, University of Oxford, Oxford, United Kingdom, and approved June 14, 2005 (received for review March 29, 2005) The main obstacle for the evolution of cooperation is that natural natural to include a complexity cost for TFT (14): the payoff selection favors defection in most settings. In the repeated pris- for TFT is reduced by a small constant c. The payoff matrix is oner’s dilemma, two individuals interact several times, and, in each given by round, they have a choice between cooperation and defection. We analyze the evolutionary dynamics of three simple strategies ALLC ALLD TFT for the repeated prisoner’s dilemma: always defect (ALLD), al- ALLC Rm Sm Rm ways cooperate (ALLC), and tit-for-tat (TFT). We study mutation– ALLD ͩ Tm Pm T ϩ P͑m Ϫ 1͒ͪ . selection dynamics in finite populations. Despite ALLD being the TFT Rm Ϫ cSϩ P͑m Ϫ 1͒ Ϫ cRmϪ c only strict Nash equilibrium, we observe evolutionary oscillations among all three strategies. The population cycles from ALLD to TFT [1] to ALLC and back to ALLD. Most surprisingly, the time average of these oscillations can be entirely concentrated on TFT. In contrast The pairwise comparison of the three strategies leads to the to the classical expectation, which is informed by deterministic following conclusions. -

Interpreting the Tape of Life: Ancestry-Based Analyses Provide
Interpreting the Tape of Life: Emily Dolson* Michigan State University Ancestry-Based Analyses Provide BEACON Center for the Study of Insights and Intuition about Evolution in Action Department of Computer Science Evolutionary Dynamics and Engineering Ecology, Evolutionary Biology, and Behavior Program [email protected] Alexander Lalejini Michigan State University Abstract Fine-scale evolutionary dynamics can be challenging to BEACON Center for the Study of tease out when focused on the broad brush strokes of whole Evolution in Action populations over long time spans. We propose a suite of diagnostic Department of Computer Science analysis techniques that operate on lineages and phylogenies in digital and Engineering evolution experiments, with the aim of improving our capacity to Ecology, Evolutionary Biology, quantitatively explore the nuances of evolutionary histories in and Behavior Program digital evolution experiments. We present three types of lineage measurements: lineage length, mutation accumulation, and phenotypic volatility. Additionally, we suggest the adoption of four Steven Jorgensen phylogeny measurements from biology: phylogenetic richness, Michigan State University phylogenetic divergence, phylogenetic regularity, and depth of the BEACON Center for the Study of most-recent common ancestor. In addition to quantitative metrics, Evolution in Action we also discuss several existing data visualizations that are useful for Department of Computer Science understanding lineages and phylogenies: state sequence visualizations, and Engineering fitness landscape overlays, phylogenetic trees, and Muller plots. We examine the behavior of these metrics (with the aid of data visualizations) in two well-studied computational contexts: (1) a set of Charles Ofria two-dimensional, real-valued optimization problems under a range of Michigan State University mutation rates and selection strengths, and (2) a set of qualitatively BEACON Center for the Study of different environments in the Avida digital evolution platform. -

Evolutionary Dynamics, Cognitive Processes, and Bayesian Inference Jordan W
TICS 1676 No. of Pages 9 Opinion Evolution in Mind: Evolutionary Dynamics, Cognitive Processes, and Bayesian Inference Jordan W. Suchow,1,* David D. Bourgin,1 and Thomas L. Griffiths1 Evolutionary theory describes the dynamics of population change in settings Trends affected by reproduction, selection, mutation, and drift. In the context of human Correspondences between the cognition, evolutionary theory is most often invoked to explain the origins of mathematics of evolution and learning capacities such as language, metacognition, and spatial reasoning, framing permit reinterpretation of evolutionary them as functional adaptations to an ancestral environment. However, evolu- processes as algorithms for Bayesian inference. tionary theory is useful for understanding the mind in a second way: as a mathematical framework for describing evolving populations of thoughts, Cognitive processes such as memory maintenance and creativity can be for- ideas, and memories within a single mind. In fact, deep correspondences exist mally modelled using the framework of between the mathematics of evolution and of learning, with perhaps the deep- evolutionary dynamics. est being an equivalence between certain evolutionary dynamics and Bayesian Maintenance in working memory can inference. This equivalence permits reinterpretation of evolutionary processes be modeled as a particle filter that as algorithms for Bayesian inference and has relevance for understanding repeatedly attempts to estimate the diverse cognitive capacities, including memory and creativity. state of the world through a sample- based approximation whose fidelity is limited by the availability of computa- Linking Evolution and Cognition tional resources. In settings as diverse as cancer biology and color vision, evolutionary dynamics provides a Creative search during problem sol- unifying mathematical framework for understanding how populations of replicators evolve ving can be seen as a stochastic when subject to mutation, selection, and random drift [1].