Home Infusion
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

References Used in Algorithms for the Treatment of Persons with Crohn’S Disease
REFERENCES USED IN ALGORITHMS FOR THE TREATMENT OF PERSONS WITH CROHN’S DISEASE 1. AA Pharma Inc: Winpred (prednisone). In: CA Product Monograph. Vaughan, ON; 2018. 2. AbbVie Corporation: Humira (adalimumab). In: CA Product Monograph. St Laurent, QC; 2019. 3. AbbVie Inc: Humira (adalimumab). In: US Product Monograph. North Chicago, IL; 2020. 4. Amgen Canada Inc: Avsola (infliximab). In: CA Product Monograph. Mississauga, ON; 2020. 5. Amgen Inc: Amjevita (adalimumab-atto). In: US Product Monograph. Thousand Oaks, CA; 2019. 6. Amgen Inc: Avsola (infliximab-axxq). In: US Product Monograph. Thousand Oaks, CA; 2019. 7. Antares Pharma Inc: Methotrexate. In: FDA Product Monograph. Ewing, NJ; 2019. 8. Apotex Inc: Methotrexate. In: CA Product Monograph. Toronto, ON; 2019. 9. Asada A, Nishida A, Shioya M, Imaeda H, Inatomi O, Bamba S, Kito K, Sugimoto M, Andoh A: NUDT15 R139C-related thiopurine leukocytopenia is mediated by 6-thioguanine nucleotide-independent mechanism in Japanese patients with inflammatory bowel disease. Journal of gastroenterology 2016, 51(1):22-29. 10. Aspen Pharmacare Canada Inc: Imuran (azathioprine). In: CA Product Monograph. Oakville, ON; 2019. 11. Biogen Canada Inc: Tysabri (natalizumab). In: CA Product Monograph. Mississauga, ON; 2017. 12. Biogen Idec Inc: Tysabri (natalizumab). In: US Product Monograph. Cambridge, MA; 2019. 13. Birdwell KA, Decker B, Barbarino JM, Peterson JF, Stein CM, Sadee W, Wang D, Vinks AA, He Y, Swen JJ et al: Clinical Pharmacogenetics Implementation Consortium (CPIC) Guidelines for CYP3A5 Genotype and Tacrolimus Dosing. Clinical pharmacology and therapeutics 2015, 98(1):19-24. 14. Boehringer Ingelheim Pharmaceuticals Inc: Cyltezo (adalimumab-adbm). In: US Product Monograph. Ridgefield, CT; 2019. -

Bio/Pharmaceutical Outsourcing Report: November 2016
Volume 21, Number 11, November 2016 Bio/Pharmaceutical Outsourcing Report Part of PharmSource STRATEGIC ADVANTAGE Business Conditions 1 Business Conditions 1 PE-Owned Companies in Line to Change Hands PE-Owned Companies in Line to Change Hands 2 Commercial Dose Manufacturing and Mergers and acquisitions are a hot topic in the contract services industry these Packaging days, and there is a lot of curiosity about which companies are likely to change 2 New Manufacturing Deal, Temporary hands. Of course, any company is a target at any time, especially when the price is Closures and Recall for Patheon right, but insight to likely acquisition candidates can be gained by looking at the list 3 Commercial Dose Manufacturing and Packaging in Brief 3 Clinical Dose Manufacturing and leastof companies four years owned ago are by likelyprivate targets equity in (PE) the nextfirms. year On oraverage, two. PE firms tend to hold Packaging onto their investments for about five years, so companies that were acquired at 3 Marken to be Acquired by UPS The table on page 2 lists 23 CMC services companies that are PE-owned; the list 5 Clinical Dose Manufacturing and was derived from the PharmSource Strategic Advantage database “Search by Packaging in Brief Ownership” feature. It is sorted by the year each company was acquired, and 6 Side Effects: Impacts of Key Events on suggests that at least 13 CMOs and CDMOs are ripe for a transaction in the foresee- CMOs and CROs able future. 7 API — Large Molecule The sale of one company, Marken (Durham, N.C., USA), was announced just this 7 Samsung BioLogics Completes IPO month (see news item, Page 3); another, Capsugel (Morristown, N.J., USA), has been 8 API — Large Molecule in Brief mentioned in the business press as being prepared for an ownership change. -

Comparison of Oral Contraceptives and Non-Oral Alternatives
PL Detail-Document #290305 −This PL Detail-Document gives subscribers additional insight related to the Recommendations published in− PHARMACIST’S LETTER / PRESCRIBER’S LETTER March 2013 Comparison of Oral Contraceptives and Non-Oral Alternatives —More information about the use of contraceptives is available in our PL Detail-Document, Hormonal Contraception— Productsa Manufacturerb Estrogen Progestin LOW-DOSE MONOPHASIC PILLS Aviane-28 Teva EE 20 mcg Levonorgestrel 0.1 mg Falmina Novast Lessina Teva Lutera Actavis Orsythia Qualitest Sronyx Actavis Gildess Fe 1/20 Qualitest EE 20 mcg Norethindrone acetate Junel 1/20 Teva 1 mg Junel Fe 1/20 Teva Loestrin-21 1/20 Warner Chilcott/Teva Loestrin Fe 1/20 Warner Chilcott/Teva Microgestin 1/20 Actavis Microgestin Fe 1/20 Actavis Generess Fe chewable Actavis EE 25 mcg Norethindrone 0.8 mg Altavera Sandoz EE 30 mcg Levonorgestrel 0.15 mg Kurvelo Lupin Levora Actavis Marlissa Glenmark Nordette-28 Duramed/Teva Portia-28 Teva Cryselle-28 Teva EE 30 mcg Norgestrel 0.3 mg Elinest Novast Low-Ogestrel-21 Actavis Low-Ogestrel-28 Actavis Lo/Ovral-28 Wyeth Gildess Fe 1.5/30 Qualitest EE 30 mcg Norethindrone acetate Junel 1.5/30 Teva 1.5 mg Junel Fe 1.5/30 Teva Loestrin 1.5/30-21 Warner Chilcott/Teva Loestrin Fe 1.5/30 Warner Chilcott/Teva Microgestin 1.5/30 Actavis Microgestin Fe 1.5/30 Actavis More. Copyright © 2013 by Therapeutic Research Center 3120 W. March Lane, Stockton, CA 95219 ~ Phone: 209-472-2240 ~ Fax: 209-472-2249 www.PharmacistsLetter.com ~ www.PrescribersLetter.com ~ www.PharmacyTechniciansLetter.com -
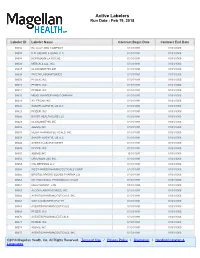
Active Labelers Run Date : Feb 19, 2018
Active Labelers Run Date : Feb 19, 2018 Labeler ID Labeler Name Contract Begin Date Contract End Date 00002 ELI LILLY AND COMPANY 01/01/1991 01/01/3000 00003 E.R. SQUIBB & SONS, LLC. 01/01/1991 01/01/3000 00004 HOFFMANN-LA ROCHE 01/01/1991 01/01/3000 00006 MERCK & CO., INC. 01/01/1991 01/01/3000 00007 GLAXOSMITHKLINE 01/01/1991 01/01/3000 00008 WYETH LABORATORIES 01/01/1991 01/01/3000 00009 PFIZER, INC 01/01/1991 01/01/3000 00013 PFIZER, INC. 01/01/1991 01/01/3000 00014 PFIZER, INC 01/01/1991 01/01/3000 00015 MEAD JOHNSON AND COMPANY 01/01/1991 01/01/3000 00023 ALLERGAN INC 01/01/1991 01/01/3000 00024 SANOFI-AVENTIS, US LLC 01/01/1991 01/01/3000 00025 PFIZER, INC. 01/01/1991 01/01/3000 00026 BAYER HEALTHCARE LLC 01/01/1991 01/01/3000 00029 GLAXOSMITHKLINE 01/01/1991 01/01/3000 00032 ABBVIE INC. 01/01/1991 01/01/3000 00037 MEDA PHARMACEUTICALS, INC. 01/01/1991 01/01/3000 00039 SANOFI-AVENTIS, US LLC 01/01/1991 01/01/3000 00046 AYERST LABORATORIES 01/01/1991 01/01/3000 00049 PFIZER, INC 01/01/1991 01/01/3000 00051 ABBVIE INC 10/01/1997 01/01/3000 00052 ORGANON USA INC. 01/01/1991 01/01/3000 00053 CSL BEHRING LLC 01/01/1991 01/01/3000 00054 WEST-WARD PHARMACEUTICALS CORP. 01/01/1991 01/01/3000 00056 BRISTOL-MYERS SQUIBB PHARMA CO. 01/01/1991 01/01/3000 00062 ORTHO MCNEIL PHARMACEUTICALS 01/01/1991 01/01/3000 00064 HEALTHPOINT, LTD. -

Long Term Care Pharmacy
2201812 Long Term Care Pharmacy Long-Term Care Pharmacy of charge as value-added services. They include: Our mission is to provide our long-term care pharmacy › Access to our staff of eight in-house clinical experts. members with the tools they need to maximize savings › CE credits through a monthly teleconference program, while enhancing the quality of care they provide. In 1993, in-person programming, and written education Innovatix began as a pharmacy-focused GPO, and over programs. the years has become the industry leader with over 15,000 › Care Solutions manuals that serve as management unique NDCs and a number of pharmacy support products guides for specific diseases and conditions. and services. As a wholly-owned subsidiary of Premier, › Contract Advantage tools that help members save by Innovatix also offers members access to one of the most providing contracted alternatives to higher-priced non- robust, competitive equipment, supply, and service contracted drugs. portfolios available. › Updated clinical news and resources. GPO Support Services Multi-Tiered Customer Support At Innovatix, we realize that securing best-in-class pricing Innovatix members receive ongoing support from a is only half of the equation. That’s why we’ve developed team of experienced professionals who are committed a suite of tools and services designed to ensure our to delivering exceptional value and complete member members receive discounted pricing and have sufficient satisfaction. Our customer care teams work with each data to make informed purchasing decisions. Our tools member individually, analyzing data to identify purchasing and services include: needs and goals. Our objective is to secure the greatest › Electronic contract attachment technology designed to value for each of our members. -

Pharmaceutical Company Contact Information (PDF)
Pharmaceutical Company Contact Information - Rebate Filing - as of June 2018 Labeler Name Invoice Contact Phone Extension 00002 LILLY USA, LLC LISA NORTON (317) 276-2000 00003 ER SQUIBB AND SONS INC. LYNN LEWIS (609) 897-4731 00004 GENENTECH CONTRACT ADMINISTRATION (650) 866-2666 00005 LEDERLE LABORATORIES DAN MAGUIRE (484) 563-5097 00006 MERCK & CO., INC. DOUG BICKFORD (215) 652-0671 00007 SMITHKLINE BEECHAM DAVID BUCKLEY (215) 751-5690 00008 WYETH LABORATORIES JENNIFER WOOTEN (901) 215-1883 00009 PHARMACIA AND UPJOHN COMPANY/PFIZER JENNIFER WOOTEN (901) 215-1883 00013 PHARMACIA AND UPJOHN COMPANY NICHOLAS CHRISTODOULOU (336) 291-1053 00014 G. D. SEARLE & CO. CINDY MCDONALD (847) 581-5726 00015 MEAD JOHNSON AND COMPANY LYNN LEWIS (609) 897-4731 00016 PHARMACIA INC. BARBARA WINGET (908) 901-7254 00023 ALLERGAN INC SHOBHANA MINAWALA (714) 246-6205 00024 SANOFI WINTHROP PHARMACEUTICALS LAURIE DUNLAP, ADMIN., GOVT. OPERATIONS (212) 551-4198 00025 PHARMACIA CORPORATION NICHOLAS CHRISTODOULOU (336) 291-1053 00026 BAYER CORPORATION PHARMACEUTICAL DIV. LINDA WOLCHESKI (203) 812-6372 00028 NOVARTIS PHARMACEUTICALS (862) 778-8094 00029 SMITHKLINE BEECHAM DAVID BUCKLEY (215) 751-5690 00031 A. H. ROBINS COMPANY DAN MAGUIRE (610) 902-3222 00032 SOLVAY PHARMACEUTICALS STACEY LENOX (847) 937-3979 00033 SYNTEX LABORATORIES, INC. JANICE BRENNAN (973) 562-3494 00034 THE PURDUE FREDERICK COMPANY JUNE STOWE (203) 899-8035 00037 CARTER-WALLACE, INC. JAY R BRENNAN (609) 655-6163 00038 ASTRAZENECA LP DAVID WRIGHT (302) 886-2268 7820 00039 AVENTIS PHARMACEUTICALS (908) 981-7461 00043 NOVARTIS CONSUMER HEALTH, INC. EDWARD D. COLLINS (973) 781-6191 00044 KNOLL LABORATORIES DEBRA DEYOUNG (847) 937-4372 00045 MCNEIL PHARMACEUTICAL (908) 218-6777 00046 AYERST LABORATORIES (901) 215-1473 00047 WARNER CHILCOTT LABORATORIES LISA KAROLCHYK (973) 442-3262 00048 KNOLL PHARMACEUTICAL COMPANY DEBRA DEYOUNG (847) 937-4372 00049 ROERIG NICHOLAS CHRISTODOULOU (336) 291-1053 00051 UNIMED PHARMACEUTICALS, INC STACY LENOX (847) 937-3979 00052 ORGANON, USA, INC. -

United States District Court for the Western District of Kentucky Louisville Division
Case 3:18-cv-00558-CRS Document 1 Filed 08/17/18 Page 1 of 299 PageID #: 1 UNITED STATES DISTRICT COURT FOR THE WESTERN DISTRICT OF KENTUCKY LOUISVILLE DIVISION BAPTIST HEALTHCARE SYSTEM, INC. d/b/a BAPTIST HEALTH CORBIN, BAPTIST HEALTH LA GRANGE, BAPTIST HEALTH LEXINGTON, COMPLAINT BAPTIST HEALTH LOUISVILLE, BAPTIST HEALTH PADUCAH, and CASE NO. _______________________3:18-cv-558-CHB BAPTIST HEALTH FLOYD; and JURY TRIAL DEMANDED BAPTIST HEALTH MADISONVILLE, INC. d/b/a BAPTIST HEALTH MADISONVILLE; This Action Relates to: Case No. 1:17-MD-2804 and Hon. Dan A. Polster BAPTIST HEALTH RICHMOND, INC. d/b/a BAPTIST HEALTH RICHMOND; Under: and Organized Crime Control Act of 1970, IX, BOWLING GREEN WARREN COUNTY Racketeer Influenced and Corrupt COMMUNITY HOSPITAL Organizations Act, P.L. No. 91-452, 84 CORPORATION d/b/a THE MEDICAL State. 922 (1970), (codified at 18 U.S.C. §§ CENTER AT BOWLING GREEN, THE 1961-1967 (2012) (“RICO”) MEDICAL CENTER AT CAVERNA, AND THE MEDICAL CENTER AT Breach of Implied Warranty of Fitness for SCOTTSVILLE; a Particular Purpose, KRS 355.2-315 and Kentucky Consumer Protection Act, KRS 367.170 THE MEDICAL CENTER AT CLINTON Negligence COUNTY, INC. d/b/a THE MEDICAL CENTER AT ALBANY Wanton Negligence and Negligence Per Se THE MEDICAL CENTER AT Negligent Marketing FRANKLIN, INC. d/b/a THE MEDICAL CENTER AT FRANKLIN Negligent Distribution Plaintiffs, Nuisance v. Unjust Enrichment AMERISOURCEBERGEN DRUG CORPORATION; Case 3:18-cv-00558-CRS Document 1 Filed 08/17/18 Page 2 of 299 PageID #: 2 CARDINAL HEALTH, INC.; MCKESSON CORPORATION; PURDUE PHARMA L.P.; PURDUE PHARMA, INC.; THE PURDUE FREDERICK COMPANY, INC.; TEVA PHARMACEUTICAL INDUSTRIES, LTD.; TEVA PHARMACEUTICALS USA, INC.; CEPHALON, INC.; JOHNSON & JOHNSON; JANSSEN PHARMACEUTICALS, INC.; ORTHO-MCNEIL-JANSSEN PHARMACEUTICALS, INC. -

05/09/2016 Provider Subsystem Healthcare and Family Services Run Time: 04:25:50 Report Id 2794D052 Page: 01
MEDICAID SYSTEM (MMIS) ILLINOIS DEPARTMENT OF RUN DATE: 05/09/2016 PROVIDER SUBSYSTEM HEALTHCARE AND FAMILY SERVICES RUN TIME: 04:25:50 REPORT ID 2794D052 PAGE: 01 ALPHA COMPLETE LIST OF PHARMACEUTICAL LABELERS WITH SIGNED REBATE AGREEMENTS IN EFFECT AS OF 07/01/2016 NDC NDC PREFIX LABELER NAME PREFIX LABELER NAME 68782 (OSI) EYETECH 55513 AMGEN USA 00074 ABBOTT LABORATORIES 58406 AMGEN/IMMUNEX 68817 ABRAXIS BIOSCIENCE, LLC 53746 AMNEAL PHARMACEUTICALS 16729 ACCORD HEALTHCARE INCORPORATED 65162 AMNEAL PHARMACEUTICALS LLC 42192 ACELLA PHARMACEUTICALS, LLC 69238 AMNEAL PHARMACEUTICALS, LLC 10144 ACORDA THERAPEUTICS, INC. 53150 AMNEAL-AGILA, LLC 00472 ACTAVIS 00548 AMPHASTAR PHARMACEUTICALS, INC. 00228 ACTAVIS ELIZABETH LLC 69918 AMRING PHARMACEUTICALS, INC. 45963 ACTAVIS INC. 66780 AMYLIN PHARMACEUTICALS, INC. 46987 ACTAVIS KADIAN LLC 55724 ANACOR PHARMACEUTICALS 49687 ACTAVIS KADIAN LLC 10370 ANCHEN PHARMACEUTICALS, INC. 14550 ACTAVIS PHARMA MFGING PRIVATE LIMITED 43595 ANGELINI PHARMA, INC. 61874 ACTAVIS PHARMA, INC. 62559 ANIP ACQUISITION COMPANY 67767 ACTAVIS SOUTH ATLANTIC 54436 ANTARES PHARMA, INC. 66215 ACTELION PHARMACEUTICALS U.S., INC. 52609 APO-PHARMA USA, INC. 52244 ACTIENT PHARMACEUTICALS 60505 APOTEX CORP. 75989 ACTON PHARMACEUTICALS 63323 APP PHARMACEUTICALS, LLC. 69547 ADAPT PHARMA INC. 43485 APRECIA PHARMACEUTICALS COMPANY 76431 AEGERION PHARMACEUTICALS, INC. 42865 APTALIS PHARMA US, INC 50102 AFAXYS, INC. 58914 APTALIS PHARMA US, INC. 10572 AFFORDABLE PHARMACEUTICALS, LLC 13310 AR SCIENTIFIC, INC. 27241 AJANTA PHARMA LIMITED 08221 ARBOR PHARM IRELAND LIMITED 17478 AKORN INC 60631 ARBOR PHARMACEUTICALS IRELAND LIMITED 24090 AKRIMAX PHARMACEUTICALS LLC 24338 ARBOR PHARMACEUTICALS, INC. 68220 ALAVEN PHARMACEUTICAL, LLC 59923 AREVA PHARMACEUTICALS 00065 ALCON LABORATORIES, INC. 76189 ARIAD PHARMACEUTICALS, INC. 00998 ALCON LABORATORIES, INC. 24486 ARISTOS PHARMACEUTICALS, INC. -

Rebateable Manufacturers
Rebateable Labelers – July 2021 Manufacturers are responsible for updating their eligible drugs and pricing with CMS. Montana Healthcare Programs will not pay for an NDC not updated with CMS. Note: Some manufacturers on this list may have some NDCs that are covered and others that are not. Manufacturer ID Manufacturer Name 00002 ELI LILLY AND COMPANY 00003 E.R. SQUIBB & SONS, LLC. 00004 HOFFMANN-LA ROCHE 00006 MERCK & CO., INC. 00007 GLAXOSMITHKLINE 00008 WYETH PHARMACEUTICALS LLC, 00009 PHARMACIA AND UPJOHN COMPANY LLC 00013 PFIZER LABORATORIES DIV PFIZER INC 00015 MEAD JOHNSON AND COMPANY 00023 ALLERGAN INC 00024 SANOFI-AVENTIS, US LLC 00025 PFIZER LABORATORIES DIV PFIZER INC 00026 BAYER HEALTHCARE LLC 00032 ABBVIE INC. 00037 MEDA PHARMACEUTICALS, INC. 00039 SANOFI-AVENTIS, US LLC 00046 WYETH PHARMACEUTICALS INC. 00049 ROERIG 00051 ABBVIE INC 00052 ORGANON USA INC. 00053 CSL BEHRING L.L.C. 00054 HIKMA PHARMACEUTICAL USA, INC. 00056 BRISTOL-MYERS SQUIBB PHARMA CO. 00065 ALCON LABORATORIES, INC. 00068 AVENTIS PHARMACEUTICALS 00069 PFIZER LABORATORIES DIV PFIZER INC 00071 PARKE-DAVIS DIV OF PFIZER 00074 ABBVIE INC 00075 AVENTIS PHARMACEUTICALS, INC. 00078 NOVARTIS 00085 SCHERING CORPORATION 00087 BRISTOL-MYERS SQUIBB COMPANY 00088 AVENTIS PHARMACEUTICALS 00093 TEVA PHARMACEUTICALS USA, INC. 00095 BAUSCH HEALTH US, LLC Page 1 of 19 Manufacturer ID Manufacturer Name 00096 PERSON & COVEY, INC. 00113 L. PERRIGO COMPANY 00115 IMPAX GENERICS 00116 XTTRIUM LABORATORIES, INC. 00121 PHARMACEUTICAL ASSOCIATES, INC. 00131 UCB, INC. 00132 C B FLEET COMPANY INC 00143 HIKMA PHARMACEUTICAL USA, INC. 00145 STIEFEL LABORATORIES, INC, 00168 E FOUGERA AND CO. 00169 NOVO NORDISK, INC. 00172 TEVA PHARMACEUTICALS USA, INC 00173 GLAXOSMITHKLINE 00178 MISSION PHARMACAL COMPANY 00185 EON LABS, INC. -

Strayhorn V. Wyeth Pharmaceuticals Inc
Case 2:11-cv-02058-STA-cgc Document 112 Filed 08/08/12 Page 1 of 22 PageID 2694 IN THE UNITED STATES DISTRICT COURT FOR THE WESTERN DISTRICT OF TENNESSEE WESTERN DIVISION ______________________________________________________________________________ GLORIA STRAYHORN and JEREMY ) STRAYHORN, ) ) Plaintiffs, ) ) v. ) No. 11-2058-STA-cgc ) WYETH PHARMACEUTICALS, INC.; ) WYETH LLC; WYETH, INC.; PFIZER, ) INC.; SCHWARZ PHARMA, INC.; ) SCHWARZ PHARMA AG; UCB GmbH; ) ALAVEN PHARMACEUTICALS LLC; ) and ACTAVIS ELIZABETH LLC, ) ) Defendants. ) ______________________________________________________________________________ SARAH SPEED, ) ) Plaintiff, ) ) v. ) No. 11-2095-STA-cgc ) WYETH PHARMACEUTICALS, INC.; ) WYETH LLC; WYETH, INC.; PFIZER, ) INC.; SCHWARZ PHARMA, INC.; and ) WATSON LABORATORIES, INC., ) ) Defendants. ) ______________________________________________________________________________ KATHLEEN SIMMONS, ) ) Plaintiff, ) ) v. ) No. 11-2083-STA-cgc ) WYETH PHARMACEUTICALS, INC.; ) 1 Case 2:11-cv-02058-STA-cgc Document 112 Filed 08/08/12 Page 2 of 22 PageID 2695 WYETH LLC; WYETH, INC.; PFIZER, ) INC.; SCHWARZ PHARMA, INC.; ) ALAVEN PHARMACEUTICAL, LLC; ) and WATSON LABORATORIES, INC., ) ) Defendants. ) ______________________________________________________________________________ GORDON and JUDITH WEAVER; ) SHENA JOHNSON; DEAN BROWN; ) GWENDOLYN RUFF; EMMA ) KETRON; LARRY HUDSON; ANNA ) ODOM; MARILYN MONCIER; ) THELMA DONALD; NETTER ) GRIGGS; ORVIELL RHODES; SELMA ) CARTER; and GERTIE KING, ) ) Plaintiffs, ) ) v. ) No. 11-2134-STA-cgc ) -

Convention Exhibitors and Sponsors—October 2007
CONVENTION EXHIBITORS /alert Marketing Drug Enforcement Innovation Outcomes Pharmaceutical Abbott Administration Innovatix HealthCare Abbott Diabetes Care Drug Topics InterCure Owen Mumford Acorda Therapeutics Duramed Pharmaceuticals, Inc. Janssen L.P. PAAS National Adams Respiratory Therapeutics Eagle Health Supplies, Inc. Jascorp Pacific Pharmacy Computers Aetrex Worldwide, Inc. ECRS Kelli’s Gift Shop Suppliers Package Express Center, Inc. AIMSCO/Delta Hi-Tech, Inc. EISAI KeyCentrix Inc. Paddock Laboratories, Inc. Alpharma Eli Lilly & Company Kinray Inc. Pakor Inc. American Lifeline Emdeon Business Services Kirby Lester, LLC Parata Systems American Pharmacists Emporos Systems Lexi-Comp ParMed Pharmaceuticals Association Endo Pharmaceuticals Inc. Liberty Photo Products Partners in Pharmacy American Society of EPIC Pharmacies Inc. Life Line Screening PBA /TrueCare Pharmacies Consultant Pharmacists eRx Network, LLC Life-File LLC PDQ Communications Inc. AmerisourceBergen ETHEX Corporation LifeScan Inc. PDX-RX.com-PCI-FDS Corporation EXP Pharmaceutical Managed Health Care Pharmacist e-link Anda Inc. Services Corp. Associates Inc. Pharmacists Mutual Companies Apotex Corporation FDS, Inc. Mason Vitamins Inc. Pharmacists OnLine Apothecary Products Inc. Federation of Pharmacy Masters Pharmaceutical, a Pharmacy Choice, Inc. Apothecary Rx Networks Div. of DBS Trading Inc. Pharmacy Consulting Associates Associated Pharmacies, Inc. Fillmaster Systems LLC Maxim Staffing Solutions Pharmacy Development Services Astellas Pharma US Inc. First DataBank McKesson Pharmacy First/Wholesale AstraZeneca Flavorx McQueary Brothers Drug Co. Alliance LLC Ateb, Inc. G & W Laboratories, Inc. Meadowbrook Insurance Group Pharmacy Times Auburn Pharmaceutical G+M North America, Inc. Medical Matrix LP Pharmex, a Div of Time Auxilium Pharmaceuticals, Inc. Gallipot Inc. Medicine Shoppe Med Labeling Bayer Healthcare GeriMed/Rx Med/IV Med International, Inc. Pill Box, The Pharmaceuticals Gifts for Medical Professionals Medisca Inc. -

Lou Schmukler of BMS on Creating Supply Chain Excellence
The Official Magazine of ISPE September-October 2016 | Volume 36, Number 5 Lou Schmukler of BMS on Creating Supply Chain Excellence How to Fight a Bully: An Interview with Nicole Pierson Meet Your 2016-2017 Board of Directors Quarterly Report: BIOTECHNOLOGY www.PharmaceuticalEngineering.org Pharmaceutical Engineering | September-October 2016 | 1 WHY WE DO IT CLEAN AIR MATTERS camfilapc.com/cleanairmatters Farr Gold Series® Camtain Dust Collector Quad Pulse Package Dust Collector Dust, Mist and Fume Collectors AIR POLLUTION CONTROL www.camfilapc.com • e-mail: [email protected] • 855-405-2271 CHANGE IS OPPORTUNITY Some see change as a problem; we see change as an opportunity. Adapting to the evolving trends and ever- changing regulations in the life sciences industry is what we’re known for. We’re driven to find the right solution to the most technically challenging problems. And we’re satisfied only when we’ve produced results that make you successful. crbusa.com THE RELENTLESS PURSUIT OF SUCCESS. YOURS .™ Biological Animal Health OSD Blood Fractionation Fill/Finish Oligonucleo/Peptides Vaccines Medical Devices API’s Nutraceuticals Engineering | Architecture | Construction | Consulting Editor’s Voice A First Time for Everything My introduction to the world of biopharma occurred in June 2015 as I listened to Andy Skibo, then Chair of ISPE’s Board, deliver his “Biologics Supply Chain Risks” keynote address at the ISPE/FDA/PQRI Editorial Board Quality Manufacturing Conference in Washington, DC. He emphasized the potential effect of supply Editor in chief: Anna Maria di Giorgio chain risk on biologics, which are projected to account for 80% of the pipeline and 70% of sales revenue Managing editor: Amy Loerch by 2020—just three short years away.