Microfabricated Thin-Film Batteries: Technology and Potential Applications
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Electrolyte Panel
Lab Dept: Chemistry Test Name: ELECTROLYTE PANEL General Information Lab Order Codes: LYTE Synonyms: Electrolytes; Lytes CPT Codes: 80051 – Electrolyte panel Test Includes: Carbon Dioxide, Chloride, Potassium, Sodium concentrations reported in mEq/L, and AGAP (Anion Gap). Logistics Test Indications: The maintenance of osmotic pressure and water distribution in the various body fluid compartments is primarily a function of the four major electrolytes, Na+, K+, Cl-, and HCO3-. In addition to water homeostasis, these electrolytes play an important part in the maintenance of pH, regulation of proper heart and muscle functions, involvement in electron transfer reactions, and participation in catalysis as cofactors for enzymes. Lab Testing Sections: Chemistry Phone Numbers: MIN Lab: 612-813-6280 STP Lab: 651-220-6550 Test Availability: Daily, 24 hours Turnaround Time: 30 minutes Special Instructions: N/A Specimen Specimen Type: Blood Container: Green top (Li Heparin) tube, preferred Alternate tube: Red, marble or gold top tube Draw Volume: 0.6 mL blood Processed Volume: 0.2 mL serum/plasma Collection: Routine blood collection. Mix tubes containing anticoagulant by gentle inversion. Note: Venipuncture samples are preferred, but capillary specimens will be accepted. Special Processing: Lab Staff: Sample must be run within one hour of collection for CO2 stability. Centrifuge specimen, remove serum/plasma aliquot into a plastic sample cup. Avoid prolonged contact with separated cells. Store at refrigerated temperatures. Patient Preparation: None Sample Rejection: Mislabeled or unlabeled specimens Interpretive Reference Range: See individual analyte procedures Critical Values: See individual analyte procedures Limitations: See individual analyte procedures Methodology: See individual analyte procedures References: See individual analyte procedures Updates: 2/8/2016: Update alt tube types . -

Electrochemical Cells
Electrochemical cells = electronic conductor If two different + surrounding electrolytes are used: electrolyte electrode compartment Galvanic cell: electrochemical cell in which electricity is produced as a result of a spontaneous reaction (e.g., batteries, fuel cells, electric fish!) Electrolytic cell: electrochemical cell in which a non-spontaneous reaction is driven by an external source of current Nils Walter: Chem 260 Reactions at electrodes: Half-reactions Redox reactions: Reactions in which electrons are transferred from one species to another +II -II 00+IV -II → E.g., CuS(s) + O2(g) Cu(s) + SO2(g) reduced oxidized Any redox reactions can be expressed as the difference between two reduction half-reactions in which e- are taken up Reduction of Cu2+: Cu2+(aq) + 2e- → Cu(s) Reduction of Zn2+: Zn2+(aq) + 2e- → Zn(s) Difference: Cu2+(aq) + Zn(s) → Cu(s) + Zn2+(aq) - + - → 2+ More complex: MnO4 (aq) + 8H + 5e Mn (aq) + 4H2O(l) Half-reactions are only a formal way of writing a redox reaction Nils Walter: Chem 260 Carrying the concept further Reduction of Cu2+: Cu2+(aq) + 2e- → Cu(s) In general: redox couple Ox/Red, half-reaction Ox + νe- → Red Any reaction can be expressed in redox half-reactions: + - → 2 H (aq) + 2e H2(g, pf) + - → 2 H (aq) + 2e H2(g, pi) → Expansion of gas: H2(g, pi) H2(g, pf) AgCl(s) + e- → Ag(s) + Cl-(aq) Ag+(aq) + e- → Ag(s) Dissolution of a sparingly soluble salt: AgCl(s) → Ag+(aq) + Cl-(aq) − 1 1 Reaction quotients: Q = a − ≈ [Cl ] Q = ≈ Cl + a + [Ag ] Ag Nils Walter: Chem 260 Reactions at electrodes Galvanic cell: -

3 PRACTICAL APPLICATION BATTERIES and ELECTROLYSIS Dr
ELECTROCHEMISTRY – 3 PRACTICAL APPLICATION BATTERIES AND ELECTROLYSIS Dr. Sapna Gupta ELECTROCHEMICAL CELLS An electrochemical cell is a system consisting of electrodes that dip into an electrolyte and in which a chemical reaction either uses or generates an electric current. A voltaic or galvanic cell is an electrochemical cell in which a spontaneous reaction generates an electric current. An electrolytic cell is an electrochemical cell in which an electric current drives an otherwise nonspontaneous reaction. Dr. Sapna Gupta/Electrochemistry - Applications 2 GALVANIC CELLS • Galvanic cell - the experimental apparatus for generating electricity through the use of a spontaneous reaction • Electrodes • Anode (oxidation) • Cathode (reduction) • Half-cell - combination of container, electrode and solution • Salt bridge - conducting medium through which the cations and anions can move from one half-cell to the other. • Ion migration • Cations – migrate toward the cathode • Anions – migrate toward the anode • Cell potential (Ecell) – difference in electrical potential between the anode and cathode • Concentration dependent • Temperature dependent • Determined by nature of reactants Dr. Sapna Gupta/Electrochemistry - Applications 3 BATTERIES • A battery is a galvanic cell, or a series of cells connected that can be used to deliver a self-contained source of direct electric current. • Dry Cells and Alkaline Batteries • no fluid components • Zn container in contact with MnO2 and an electrolyte Dr. Sapna Gupta/Electrochemistry - Applications 4 ALKALINE CELL • Common watch batteries − − Anode: Zn(s) + 2OH (aq) Zn(OH)2(s) + 2e − − Cathode: 2MnO2(s) + H2O(l) + 2e Mn2O3(s) + 2OH (aq) This cell performs better under current drain and in cold weather. It isn’t truly “dry” but rather uses an aqueous paste. -

Elements of Electrochemistry
Page 1 of 8 Chem 201 Winter 2006 ELEM ENTS OF ELEC TROCHEMIS TRY I. Introduction A. A number of analytical techniques are based upon oxidation-reduction reactions. B. Examples of these techniques would include: 1. Determinations of Keq and oxidation-reduction midpoint potentials. 2. Determination of analytes by oxidation-reductions titrations. 3. Ion-specific electrodes (e.g., pH electrodes, etc.) 4. Gas-sensing probes. 5. Electrogravimetric analysis: oxidizing or reducing analytes to a known product and weighing the amount produced 6. Coulometric analysis: measuring the quantity of electrons required to reduce/oxidize an analyte II. Terminology A. Reduction: the gaining of electrons B. Oxidation: the loss of electrons C. Reducing agent (reductant): species that donates electrons to reduce another reagent. (The reducing agent get oxidized.) D. Oxidizing agent (oxidant): species that accepts electrons to oxidize another species. (The oxidizing agent gets reduced.) E. Oxidation-reduction reaction (redox reaction): a reaction in which electrons are transferred from one reactant to another. 1. For example, the reduction of cerium(IV) by iron(II): Ce4+ + Fe2+ ! Ce3+ + Fe3+ a. The reduction half-reaction is given by: Ce4+ + e- ! Ce3+ b. The oxidation half-reaction is given by: Fe2+ ! e- + Fe3+ 2. The half-reactions are the overall reaction broken down into oxidation and reduction steps. 3. Half-reactions cannot occur independently, but are used conceptually to simplify understanding and balancing the equations. III. Rules for Balancing Oxidation-Reduction Reactions A. Write out half-reaction "skeletons." Page 2 of 8 Chem 201 Winter 2006 + - B. Balance the half-reactions by adding H , OH or H2O as needed, maintaining electrical neutrality. -

A Review of Cathode and Anode Materials for Lithium-Ion Batteries
A Review of Cathode and Anode Materials for Lithium-Ion Batteries Yemeserach Mekonnen Aditya Sundararajan Arif I. Sarwat IEEE Student Member IEEE Student Member IEEE Member Department of Electrical & Department of Electrical & Department of Electrical & Computer Engineering Computer Engineering Computer Engineering Florida International University Florida International University Florida International University Email: [email protected] Email: [email protected] Email: [email protected] Abstract—Lithium ion batteries are one of the most technologies such as plug-in HEVs. For greater application use, commercially sought after energy storages today. Their batteries are usually expensive and heavy. Li-ion and Li- based application widely spans from Electric Vehicle (EV) to portable batteries show promising advantages in creating smaller, devices. Their lightness and high energy density makes them lighter and cheaper battery storage for such high-end commercially viable. More research is being conducted to better applications [18]. As a result, these batteries are widely used in select the materials for the anode and cathode parts of Lithium (Li) ion cell. This paper presents a comprehensive review of the common consumer electronics and account for higher sale existing and potential developments in the materials used for the worldwide [2]. Lithium, as the most electropositive element making of the best cathodes, anodes and electrolytes for the Li- and the lightest metal, is a unique element for the design of ion batteries such that maximum efficiency can be tapped. higher density energy storage systems. The discovery of Observed challenges in selecting the right set of materials is also different inorganic compounds that react with alkali metals in a described in detail. -

Advances in Materials Design for All-Solid-State Batteries: from Bulk to Thin Films
applied sciences Review Advances in Materials Design for All-Solid-state Batteries: From Bulk to Thin Films Gene Yang 1, Corey Abraham 2, Yuxi Ma 1, Myoungseok Lee 1, Evan Helfrick 1, Dahyun Oh 2,* and Dongkyu Lee 1,* 1 Department of Mechanical Engineering, College of Engineering and Computing, University of South Carolina, Columbia, SC 29208, USA; [email protected] (G.Y.); [email protected] (Y.M.); [email protected] (M.L.); [email protected] (E.H.) 2 Chemical and Materials Engineering Department, Charles W. Davidson College of Engineering, San José State University, San José, CA 95192-0080, USA; [email protected] * Correspondence: [email protected] (D.O.); [email protected] (D.L.) Received: 15 June 2020; Accepted: 7 July 2020; Published: 9 July 2020 Featured Application: All solid-state lithium batteries, all solid-state thin-film lithium batteries. Abstract: All-solid-state batteries (SSBs) are one of the most fascinating next-generation energy storage systems that can provide improved energy density and safety for a wide range of applications from portable electronics to electric vehicles. The development of SSBs was accelerated by the discovery of new materials and the design of nanostructures. In particular, advances in the growth of thin-film battery materials facilitated the development of all solid-state thin-film batteries (SSTFBs)—expanding their applications to microelectronics such as flexible devices and implantable medical devices. However, critical challenges still remain, such as low ionic conductivity of solid electrolytes, interfacial instability and difficulty in controlling thin-film growth. In this review, we discuss the evolution of electrode and electrolyte materials for lithium-based batteries and their adoption in SSBs and SSTFBs. -

Advancing Focused Ion Beam Characterization for Next Generation Lithium-Ion Batteries
UC San Diego UC San Diego Electronic Theses and Dissertations Title Advancing Focused Ion Beam Characterization for Next Generation Lithium-Ion Batteries Permalink https://escholarship.org/uc/item/3sh5k04b Author Lee, Jungwoo Publication Date 2018 Peer reviewed|Thesis/dissertation eScholarship.org Powered by the California Digital Library University of California UNIVERSITY OF CALIFORNIA SAN DIEGO Advancing Focused Ion Beam Characterization for Next Generation Lithium-Ion Batteries A dissertation submitted in partial satisfaction of the requirements for the degree Doctor of Philosophy in NanoEngineering by Jungwoo Zema Lee Committee in charge: Professor Ying Shirley Meng, Chair Professor David P. Fenning Professor Eric E. Fullerton Professor Olivia A. Graeve Professor Ping Liu 2018 Copyright Jungwoo Zema Lee, 2018 All rights reserved. The Dissertation of Jungwoo Zema Lee is approved, and it is acceptable in quality and form for publication on microfilm and electronically: Chair University of California San Diego 2018 iii DEDICATION To my given and chosen family iv TABLE OF CONTENTS Signature Page ..................................................................................................................... iii Dedication ............................................................................................................................ iv Table of Contents ................................................................................................................ v List of Abbreviations ......................................................................................................... -

Solid Electrolyte Batteries
SOLID ELECTROLYTE BATTERIES John B. Goodenough and Yuhao Lu Texas Materials Institute The University of Texas at Austin Project ID: ES060 DOE Vehicle Technologies Annual Merit Review Meeting May 9-13, 2011 This presentation does not contain any proprietary or confidential information. 1 The University of Texas at Austin Overview Timeline Barriers • Project Start Date-Oct. 2009 • Lithium-ion solid electrolyte. • Project End Date- Sept. 2010 • Choice of redox couples. • Percent complete: 100% complete • Flow-through- cathode cell design Budget Partners • Funding received in FY09-FY10 – $315K • Karim Zaghib of Hydro Quebec • Funding received in FY10-FY11 – $315K 2 The University of Texas at Austin Milestones Develop and test a suitable test cell. (Jan. 10) Completed Optimize components of the cell. (Apr. 10) Completed Develop a lithium/Fe(NO3)3 cell. (July. 10) Completed Design of a new cell (Sept. 10) Completed Test flow-through cell (Jan. 11) Completed 3 The University of Texas at Austin Typical Lithium Ion Battery (LIB) Cell e- e- - Separator + Li+ Anode Li+ Cathode (Reducing Agent) (Oxidizing Agent) Li+ SEI layer Electrolyte Capacity limited by Li solid solution in cathode and loss in SEI layer Voltage limited by Eg of carbonate electrolyte. 4 The University of Texas at Austin Why Oxides? E E Co: 4s0 Co: 4s0 Co(II): t4e2 µLi 4.0 eV Co(IV): t5e0 EFC EFC (pinned) Co(III): t6e0 Co(III): t6e0 O: 2p6 O: 2p6 N(E) N(E) Li CoO (0<x<0.55) LiCoO2 1-x 2 2- + Holes form peroxide (O2) for x> 0.55 or H inserted from electrolyte. -

Chapter 13: Electrochemical Cells
March 19, 2015 Chapter 13: Electrochemical Cells electrochemical cell: any device that converts chemical energy into electrical energy, or vice versa March 19, 2015 March 19, 2015 Voltaic Cell -any device that uses a redox reaction to transform chemical potential energy into electrical energy (moving electrons) -the oxidizing agent and reducing agent are separated -each is contained in a half cell There are two half cells in a voltaic cell Cathode Anode -contains the SOA -contains the SRA -reduction reaction -oxidation takes place takes place - (-) electrode -+ electrode -anions migrate -cations migrate towards the anode towards cathode March 19, 2015 Electrons move through an external circuit from the anode to cathode Electricity is produced by the cell until one of the reactants is used up Example: A simple voltaic cell March 19, 2015 When designing half cells it is important to note the following: -each half cell needs an electrolyte and a solid conductor -the electrode and electrolyte cannot react spontaneously with each other (sometimes carbon and platinum are used as inert electrodes) March 19, 2015 There are two kinds of porous boundaries 1. Salt Bridge 2. Porous Cup · an unglazed ceramic cup · tube filled with an inert · separates solutions but electrolyte such as NaNO allows ions to pass 3 through or Na2SO4 · the ends are plugged so the solutions are separated, but ions can pass through Porous boundaries allow for ions to move between two half cells so that charge can be equalized between two half cells 2+ 2– electrolyte: Cu (aq), SO4 (aq) 2+ 2– electrolyte: Zn (aq), SO4 (aq) electrode: zinc electrode: copper March 19, 2015 Example: Metal/Ion Voltaic Cell V Co(s) Zn(s) Co2+ SO 2- 4 2+ SO 2- Zn 4 Example: A voltaic cell with an inert electrode March 19, 2015 Example Label the cathode, anode, electron movement, ion movement, and write the half reactions taking place at each half cell. -

Bipolar Nickel-Metal Hydride Battery Being Developed
Bipolar Nickel-Metal Hydride Battery Being Developed Electro Energy's bipolar nickel-metal hydride battery design layout-two parallel, 24-cell stacks. (Copyright Electro Energy; used with permission.) The NASA Lewis Research Center has contracted with Electro Energy, Inc., to develop a bipolar nickel-metal hydride battery design for energy storage on low-Earth-orbit satellites (NASA contract NAS3-27787). The objective of the bipolar nickel-metal hydride battery development program is to approach advanced battery development from a systems level while incorporating technology advances from the lightweight nickel electrode field, hydride development, and design developments from nickel-hydrogen systems. This will result in a low-volume, simplified, less-expensive battery system that is ideal for small spacecraft applications. The goals of the program are to develop a 1-kilowatt, 28-volt (V), bipolar nickel-metal hydride battery with a specific energy of 100 watt-hours per kilogram (W-hr/kg), an energy density of 250 W-hr/liter and a 5-year life in low Earth orbit at 40- percent depth-of-discharge. Electro Energy has teamed with Rhône-Poulenc, Eagle-Picher Industries, Inc., Rutgers University, and Design Automation Associates to provide a well-integrated battery design. Electro Energy is the prime contractor responsible for the overall management of the program, battery design and development, component development and testing, and cell and battery testing. Rhône-Poulenc is responsible for the metal hydride component development and improvement. Eagle-Picher is supporting component and hardware development, battery design, fabrication procedures, trade studies, and documentation. Rutgers is providing treated material for the nickel electrodes in the batteries as well as analytical support for new and cycled cell components. -
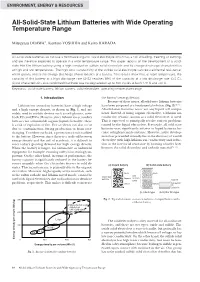
All-Solid-State Lithium Batteries with Wide Operating Temperature Range
ENVIRONMENT, ENERGY & RESOURCES All-Solid-State Lithium Batteries with Wide Operating Temperature Range Mitsuyasu OGAWA*, Kentaro YOSHIDA and Keizo HARADA All-solid-state batteries do not use a flammable organic liquid electrolyte which has a risk of boiling, freezing or burning, and are therefore expected to operate in a wide temperature range. This paper reports on the development of a solid- state thin film lithium battery using a high conductive sulfide solid electrolyte and its charge-discharge characteristics at high and low temperatures. The high ionic conductivity of the sulfide solid electrolyte can reduce internal resistance, which greatly affects the charge-discharge characteristics of a battery. Test results show that, at room temperature, the capacity of this battery at a high discharge rate (24C) reaches 89% of the capacity at a low discharge rate (0.5 C). Cycle characteristics also confirmed that there was no degradation up to 100 cycles at both 170˚C and -40˚C. Keywords: solid-state battery, lithium battery, solid electrolyte, operating temperature range 1. Introduction the battery’s energy density. Because of these issues, all-solid-state lithium batteries Lithium ion secondary batteries have a high voltage have been proposed as a fundamental solution (Fig. 2)(2)-(4). and a high energy density, as shown in Fig. 1, and are All-solid-state batteries never use any liquid cell compo- widely used in mobile devices such as cell phones, note- nents. Instead of using organic electrolyte, a lithium ion book PCs and PDAs. However, since lithium ion secondary conductive ceramic, known as a solid electrolyte, is used. -

Fluid and Electrolyte Therapy Lyon Lee DVM Phd DACVA Purposes of Fluid Administration During the Perianesthetic Period
Fluid and Electrolyte Therapy Lyon Lee DVM PhD DACVA Purposes of fluid administration during the perianesthetic period • Replace insensible fluid losses (evaporation, diffusion) during the anesthetic period • Replace sensible fluid losses (blood loss, sweating) during the anesthetic period • Maintain an adequate and effective blood volume • Maintain cardiac output and tissue perfusion • Maintain patency of an intravenous route of drug administration Review normal body water distribution • 1 gm = 1 ml; 1 kg = 1 liter; 1 kg = 2.2 lbs • Total body water: 60% of body weight • Intracellular water: 40% of body weight • Extracellular water (plasma water + interstitial water): 20% of body weight • Interstitial water: 20 % of body weight • Plasma water: 5 % of body weight • Blood volume: 9 % of body weight (blood volume = plasma water + red blood cell volume) • Inter-compartmental distribution of water is maintained by hydrostatic, oncotic, and osmotic forces • Daily water requirement: 1-3 ml/kg/hr (24-72 ml/kg/day) o 50 ml x body weight (kg) provides rough estimate for daily requirement • Requirements vary with age, environment, disease, etc… 1 Figure 1. Normal body water distribution Body 100% Water Tissue 60 % (100) 40 % Intracellular space Extracellular space 40 % (60) 20 % (40) Interstitial space Intravascular space 15 % (30) 5 % (10) Fluid movement across capillary membranes • Filtration is governed by Starling’s equation as below • Net driving pressure into the capillary = [(Pc – Pi) – (πp – πi)] o Pc = capillary hydrostatic pressure (varies from artery to vein) o Pi = interstitial hydrostatic pressure (0) o πp = plasma oncotic pressure (28 mmHg) o πi = interstitial oncotic pressure (3 mmHg) • If colloid osmotic pressure (COP) in the capillaries decreases lower than the COP in the interstitium, fluid will move out of the vessels and edema will develop.