Official Journal L 79 of the European Union
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
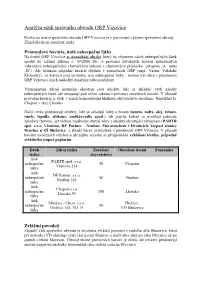
Analýza Rizik Správního Obvodu ORP Vizovice
Analýza rizik správního obvodu ORP Vizovice Riziko na území správního obvodu ORP Vizovice je v porovnání s jinými správními obvody Zlínského kraje poměrně nízké. Průmyslové havárie, únik nebezpečné látky Na území ORP Vizovice se nenachází objekt, který by objemem zásob nebezpečných látek spadal do režimu zákona č. 59/2006 Sb., o prevenci závažných havárií způsobených vybranými nebezpečnými chemickými látkami a chemickými přípravky (skupina „A“ nebo „B“). Ale účinkem případné havárie objektu v sousedících ORP (např. Vsetín, Valašské Klobouky), ve kterých jsou umístěny tyto nebezpečné látky, mohou být obce v působnosti ORP Vizovice jejich následky zasaženy nebo postiženy. Významnými zdroji možného ohrožení jsou objekty, kde se skladují větší zásoby nebezpečných látek, ale nespadají pod režim zákona o prevenci závažných havárií. V případě provozní havárie je však v jejich bezprostřední blízkosti obyvatelstvo ohroženo. Například fa. Cheport v obci Lhotsko Další rizika představují objekty, kde se skladují látky a hmoty benzin, nafta, olej, toluen, tmely, lepidla, silikony, změkčovadla apod.), při jejichž hoření se uvolňují jedovaté zplodiny (toxiny), jež mohou zasáhnout obytné zóny a objekty ohrožující výbuchem (PARTR spol. s.r.o. Všemina, DF Partner - Neubuz, Moraviachem v Hrobicích, čerpací stanice Benzina u ZŠ Slušovice a sklady barev rozmístěné v působnosti ORP Vizovice. V případě havárie uvedených objektů a dle jejího rozsahu se předpokládá vyhlášení třetího, případně zvláštního stupně poplachu. Druh Zdroj rizika Zasažené Ohrožené území Poznámka rizika obyvatelstvo únik PARTR spol. s.r.o. nebezpečné 50 Všemina Všemina 234 látky únik DF Partner, s.r.o. nebezpečné 50 Neubuz Neubuz 165 látky únik Cheport s.r.o nebezpečné 100 Lhotsko Lhotsko 93 látky únik Morávia - Chem, s.r.o. -

Commission Implementing Decision (EU) 2018/883
Status: Point in time view as at 31/10/2019. Changes to legislation: There are currently no known outstanding effects for the Commission Implementing Decision (EU) 2018/883. (See end of Document for details) Commission Implementing Decision (EU) 2018/883 of 18 June 2018 amending the Annex to Implementing Decision 2014/709/EU concerning animal health control measures relating to African swine fever in certain Member States (notified under document C(2018) 3942) (Text with EEA relevance) COMMISSION IMPLEMENTING DECISION (EU) 2018/883 of 18 June 2018 amending the Annex to Implementing Decision 2014/709/EU concerning animal health control measures relating to African swine fever in certain Member States (notified under document C(2018) 3942) (Text with EEA relevance) THE EUROPEAN COMMISSION, Having regard to the Treaty on the Functioning of the European Union, Having regard to Council Directive 89/662/EEC of 11 December 1989 concerning veterinary checks in intra-Community trade with a view to the completion of the internal market(1), and in particular Article 9(4) thereof, Having regard to Council Directive 90/425/EEC of 26 June 1990 concerning veterinary and zootechnical checks applicable in intra-Community trade in certain live animals and products with a view to the completion of the internal market(2), and in particular Article 10(4) thereof, Having regard to Council Directive 2002/99/EC of 16 December 2002 laying down the animal health rules governing the production, processing, distribution and introduction of products of animal origin for human consumption(3), and in particular Article 4(3) thereof, Whereas: (1) Commission Implementing Decision 2014/709/EU(4) lays down animal health control measures in relation to African swine fever in certain Member States, where there have been confirmed cases of that disease in domestic or feral pigs (the Member States concerned). -

Příloha Č. 2 - Seznam Zapojených Linek Do ID ZK
Příloha č. 2 - Seznam zapojených linek do ID ZK Linka Dopravce Název 1 771152 KRODOS BUS Bystřice p. Host. - Holešov - Fryšták - Zlín 2 771155 KRODOS BUS Holešov - Ludslavice - Lechotice - Otrokovice 3 771160 KRODOS BUS Kroměříž - Tlumačov - Otrokovice - Zlín 4 771161 KRODOS BUS Velké Těšany - Nová Dědina - Kvasice - Zlín 5 771201 KRODOS BUS Morkovice - Slížany - Střílky - Nemotice - Koryčany,Blišice 6 771211 KRODOS BUS Kroměříž - Rataje - Litenčice - Kunkovice 7 771231 KRODOS BUS Bystřice p. Host. - Holešov - Kroměříž 8 771240 KRODOS BUS Kroměříž - Chropyně - Záříčí 9 771241 KRODOS BUS Bystřice p. Host. - Chvalčov - Tesák - Troják 10 771242 KRODOS BUS Bystřice p.Host. - Hostýn 11 771243 KRODOS BUS Bystřice p. Host. - Všechovice - Rajnochovice 12 771244 KRODOS BUS Bystřice p. Host. - Rusava 13 771248 KRODOS BUS Holešov - Holešov,Žopy 14 771249 KRODOS BUS Holešov - Prusinovice - Bystřice p. Host. 15 771250 KRODOS BUS Holešov - Bořenovice 16 771251 KRODOS BUS Kroměříž - Hulín - Kostelec u Holešova,Karlovice 17 771252 KRODOS BUS Holešov - Kostelec u Holešova,Karlovice - Němčice 18 771255 KRODOS BUS Kroměříž - Hulín - Míškovice - Tlumačov - Kvasice 19 771261 KRODOS BUS Kroměříž - Zdounky - Zborovice - Morkovice-Slížany 20 771262 KRODOS BUS Kroměříž - Kostelany 21 771263 KRODOS BUS Kroměříž - Bařice - Velké Těšany - Lubná 22 771264 KRODOS BUS Kroměříž - Kvasice - Bělov - Karolín - Lubná 23 771271 KRODOS BUS Rusava - Holešov - Kroměříž 24 771901 KRODOS BUS Kroměříž - Střílky - Koryčany - Kyjov 25 771931 KRODOS BUS Kroměříž - Zlobice,Bojanovice - Kojetín - Prostějov 26 771933 KRODOS BUS Morkovice - Slížany - Dřínov - Věžky,Vlčí Doly 27 771934 KRODOS BUS Kroměříž - Věžky - Morkovice-Slížany - Pačlavice - Vyškov - Brno 28 771940 KRODOS BUS Kroměříž - Kyselovice - Chropyně - Přerov 29 771941 KRODOS BUS Bystřice p.Host. -

-

Celková Požadovaná
Celková požadovaná Příjmení žadatele Obec žadatele Obec realizace výše dotace Kč Abdul Vsetín Vsetín 74802 Adamec Uherský Brod Bojkovice 127500 Adamec Nezdenice Nezdenice 107500 Adámek Rožnov pod Radhoštěm Velká Lhota 127500 Adámek Rožnov pod Radhoštěm Rožnov pod Radhoštěm 127500 Adámek Jablůnka Jablůnka 107500 Adamík Ludslavice Ludslavice 90000 Adamík Lechotice Lechotice 107500 Adámková Vsetín Vsetín 102500 Adamová Zlín Hrobice 102500 Adamuška Brumov-Bylnice Brumov-Bylnice 97500 Ambruz Březolupy Březolupy 102500 Andrlík Záhorovice Záhorovice 102500 Andrys Dolní Bečva Dolní Bečva 127500 Antošová Slavičín Hostětín 120000 Arnesen Janušová Nezdenice Nezdenice 73350 Bábek Střížovice Střížovice 127500 Babíčková Provodov Prostřední Bečva 102500 Babovec Janová Janová 102500 Baček Újezd u Valašských Klobouk Újezd 87500 Baďurová Zádveřice - Raková Zádveřice-Raková 127500 Bachan Uherský Ostroh Uherský Ostroh 127500 Bachanová Uherský Ostroh Uherský Ostroh 127500 Bajza Zlín Zlín 127500 Balaštíková Tupesy Tupesy 102500 Balcar Karolinka Karolinka 102500 Balvan Valašské Meziříčí Valašské Meziříčí 127500 Bambuch Francova Lhota Francova Lhota 90000 Bambuch Kelč Kelč 127500 Bambušek Horní Bečva Horní Bečva 127500 Barcuch Slavičín Slavičín 102500 Baroň Mikulůvka Mikulůvka 107500 Barot Zlín Zlín 102500 Bártek Ratiboř Ratiboř 107500 Bartko Uherský Brod Lopeník 127500 Bartková Nivnice Nivnice 127500 Bártková Slavkov pod Hostýnem Valašské Meziříčí 102500 Bartoníková Otrokovice Otrokovice 127500 Bartošek Kudlovice Kudlovice 102500 Bartošík Slavičín Slavičín -

Saare MAAKONNA Loodusväärtused Saare MAAKONNA Loodusväärtused 2 3
SAARE MAAKONNA loodusväärtused SAARE MAAKONNA loodusväärtused 2 3 SISUKORD KAITSEALAD ................... 8 Odalätsi maastikukaitseala ....... 27 Vilsandi rahvuspark ............. 9 Panga maastikukaitseala ......... 27 Abruka looduskaitseala .......... 10 Üügu maastikukaitseala ......... 28 Laidevahe looduskaitseala ........ 11 HOIUALAD .................... 30 Liiva-Putla looduskaitseala ....... 12 Karala-Pilguse hoiuala ........... 31 Linnulaht .................... 13 Karujärve hoiuala .............. 31 Loode tammik ................ 14 Väikese väina hoiuala ........... 33 Rahuste looduskaitseala ......... 15 Viidumäe looduskaitseala ........ 16 KAITSEALUSED PARGID ........... 34 Viieristi looduskaitseala. 17 Kuressaare lossipark ............ 34 Järve luidete maastikukaitseala .... 20 Mihkel Ranna dendraarium ....... 34 Kaali maastikukaitseala .......... 20 Mõntu park .................. 35 Kaugatoma-Lõo maastikukaitseala .. 21 Pädaste park ................. 35 Kaart ....................... 22 ÜksikobjEKTID ................ 36 Kesselaiu maastikukaitseala ...... 25 Põlispuud ................... 36 Koigi maastikukaitseala .......... 25 Rändrahnud .................. 40 KAITSTAVATE LOODUSOBJEKTIDE VALITSEJA Keskkonnaamet Hiiu-Lääne-Saare regioon Tallinna 22, 93819 Kuressaare tel 452 7777 [email protected] www.keskkonnaamet.ee KAITSTAVATE LOODUSOBJEKTIDE KÜLASTUSE KORRALDAJA RMK loodushoiuosakond Viljandi mnt. 18b, 11216 Tallinn [email protected] www.rmk.ee Koostaja: Maris Sepp Trükise valmimisele aitasid kaasa: Kadri Paomees, Rein Nellis, Veljo -

Profil Města Valašské Klobouky
Profil města Valašské Klobouky Profil města Valašské Klobouky Obsah: Postavení města v rámci České republiky 4 Historie města a jeho vývoj 4 Pamětihodnosti a významní rodáci 6 Základní geografické údaje 7 Klimatické podmínky 7 Přírodní zdroje 7 ŽIVOTNÍ PROSTŘEDÍ Kvalita ovzduší 8 Kvalita vody 8 Pitná voda 10 Hlukové zatížení 11 Nakládání s odpady 11 Přírodní lokality, chráněná území 11 Veřejná zeleň 12 TECHNICKÁ INFRASTRUKTURA Zásobování vodou 13 Kanalizace a čištění odpadních vod 15 Elektrorozvody 19 Plyn 23 Tepelná energie 24 2 Veřejné osvětlení 24 Telekomunikace a radiokomunikace, internet 25 Doprava a logistické vazby 26 Dopravní obslužnost 27 Komunikace 28 LIDSKÉ ZDROJE Demografie 29 Pracovní síla 29 SOCIÁLNÍ INFRASTRUKTURA Bydlení 30 Školství 30 Zdravotnictví a sociální zabezpečení 31 Kultura 33 Cestovní ruch 34 Ekologie 35 Sport 36 MÍSTNÍ EKONOMIKA Zaměstnavatelé ve městě Valašské Klobouky 37 SPRÁVA MĚSTA Zadluženost města a vývoj dluhové služby 38 Výhled příjmů a výdajů města do roku 2005 38 3 POSTAVENÍ MĚSTA Valašské Klobouky leží v jihovýchodní části Zlínského kraje, v mikroregionu V RÁMCI ČESKÉ Jižní Valašsko. Město spadá do severní části Chráněné krajinné oblasti Bílé REPUBLIKY Karpaty. Valašské Klobouky jsou město s pověřenými úřady třetího stupně. Počet obyvatel je v současnosti 5191. Valašské Klobouky leží cca 10 km od státní hranice se Slovenskou republikou, mezi Horní Lidčí, což je poslední významný železniční uzel před hranicí se Slovenskou republikou ve Zlínském kraji a také železniční hraniční přechod, a od automobilového hraničního přechodu Brumov-Bylnice/Horné Srnie. S Horní Lidčí jsou Valašské Klobouky spojeny jak železniční tratí s pravidelným vlakovým spojením, tak silnicí první třídy I/57, která z Valašských Klobouk pokračuje do automobilového hraničního přechodu Brumova-Bylnice/Horné Srnie. -

Komentář Ke Statistickému Výkazu Regionálních Funkcí Za 1
Komentář ke statistickému výkazu regionálních funkcí okresu Zlín za rok 2015 1. PORADENSKÁ A KONZULTAČNÍ ČINNOST Počet obsluhovaných knihoven: 118 Počet poskytnutých konzultací: 526 Počet vykonaných metodických návštěv: 165 Z celkového počtu konzultací a metodických návštěv (691) bylo jednáno se starosty: 120 Vykazované číselné údaje zahrnují metodické návštěvy a konzultace v jednotlivých knihovnách, které byly zaměřeny na organizační záležitosti týkající se plnění knihovnických standardů, statistiky knihovnických činností, evidence a zpracování knihovních fondů zakoupených z finančních prostředků provozovatelů knihoven, revize a aktualizace knihovních fondů, práce s výměnným fondem, žádosti starostů o pomoc při svozu/rozvozu výměnných souborů, vykazování výkonu regionálních funkcí, vytvoření elektronické adresy knihovny, webových stránek knihoven. Během roku 2015 byly provedeny estetické úpravy, případně stěhování v těchto knihovnách: Bohuslavice nad Vláří (stěhování do nových prostor, kompletní vybavení novým nábytkem), Bohuslavice u Zlína (nový PC s tiskárnou, knihovna vymalována, nový koberec a záclona, nově natřené regály, dětský koutek s hračkami, nové uspořádání regálů), Březnice (nový PC a tiskárna, nové WC v knihovně a bezbariérový přístup), Drnovice (dvě nové skříňky na knihy a časopisy), Haluzice (nový PC), Hostišová (úprava interiéru, nové rozřaďovače, změna názvu), Hřivínův Újezd (nový PC a tiskárna), Kašava (přestěhována do nových prostor, využity vyřazené regály z Lukova), Křekov (nové hodiny, rychlovarná konvice, barevná -

Lõputöö-Hookan-Lember.Pdf (2.127Mb)
Sisekaitseakadeemia Päästekolledž Hookan Lember RS150 PÄÄSTESÜNDMUSTE ANALÜÜS PÜSIASUSTUSEGA VÄIKESAARTEL Lõputöö Juhendaja: Häli Allas MA Kaasjuhendaja: Andres Mumma Tallinn 2018 ANNOTATSIOON Päästekolledž Kaitsmine: juuni 2018 Töö pealkiri eesti keeles: Päästesündmuste analüüs püsiasustusega väikesaartel Töö pealkiri võõrkeeles: The analysis of rescue events on small islands with permanent settlements Lühikokkuvõte: Töö on kirjutatud eesti keeles ning eesti ja inglise keelse kokkuvõttega. Töö koos lisadega on kirjutatud kokku 61 lehel, millest põhiosa on 38 lehekülge. Lõputöö koosneb kolmest peatükist, kus on kasutatud kahte tabelit ja seitseteist joonist. Valitud teema uurimisprobleemiks on tervikliku ülevaate puudumine väikesaarte sündmustest, mis kuuluvad Päästeameti valdkonda. Väikesaartele toimub reageerimine erinevalt ning sõltuvalt aastaajast on reageerimine raskendatud. Ühtseid põhimõtteid rahvaarvu või sündmuste arvu kohta ei ole. Lõputöö eesmärk on analüüsida päästesündmusi väikesaartel aastatel 2009-2017 ning järeldada, millist päästevõimekust vajavad püsiasutustega väikesaared. Lõputöös antakse saartest ülevaade, mis on valimis välja toodud ning visualiseeritakse joonise abil saartel elavate püsielanike arv. Eesmärgi saavutamiseks kasutati kvantitatiivset uurimismeetodit, kus Häirekeskuselt saadud andmed korrastati ja analüüsiti. Lõputöös anti ülevaade, millised on sündmused saartel ning tehti järeldused, kuidas tagada kiire ja kvaliteetse abi kättesaadavus. Saartel, kus elanike arv on väike ning sündmuste arv minimaalne, -

1 Distribution of Dorycnium Herbaceum in the Czech Republic
Kaplan et al., Preslia 92: 255–340, 2020 Distribution of Dorycnium herbaceum in the Czech Republic Author of the map: Radomír Řepka Map produced on: 11-05-2020 Database records used for producing the distribution map of Dorycnium herbaceum published in Preslia. Coordinate system: WGS84. The mapping symbols used in the distribution map to indicate the different attributes of the occurrence in a particular grid cell. Attribute distinguished Symbol Attribute state None P all records Time P recent occurrence (at least one record since 2000) P old occurrence (all records before 2000, or demonstrably being extirpated from all localities after 2000, or all records undated) Origin P native (at least one record) × alien Source data P a revised herbarium specimen (at least one record) G all other All ? only record(s) uncertain regarding identification and/or locality 1 Kaplan et al., Preslia 92: 255–340, 2020 Abbreviations of projects (data sources): ČNFD: Czech National Phytosociological Database (managed by Department of Botany and Zoology, Faculty of Science, Masaryk University) Excerpce Atlas: Taxonomic Experts’ Records (managed by Institute of Botany, The Czech Academy of Sciences) FLDOK: Database of the Distribution of Vascular Plants in the Czech Republic (managed by Institute of Botany, The Czech Academy of Sciences) NDOP: Species Occurrence Database of NCA CR (managed by Nature Conservation Agency of the Czech Republic) The records listed below are arranged according to the grid numbers and given in their original language, usually in -

Tarif ID Zlínského Kraje
TARIF INTEGROVANÉ DOPRAVY ZLÍNSKÉHO KRAJE (ID ZK) Platnost od 01. 01. 2021 Dodatek č. 1 Tarifu ID ZK platný od 01. 09. 2021 Úplné znění, včetně všech uvedených dodatků TARIF ID ZK 2021 Obsah TARIF INTEGROVANÉ DOPRAVY ZLÍNSKÉHO KRAJE ................................................................................ 1 I. Preambule ............................................................................................................................. 3 II. Úvodní ustanovení ................................................................................................................ 3 III. Základní pojmy ...................................................................................................................... 3 IV. Druhy jízdného ...................................................................................................................... 5 V. Tarifní pravidla ...................................................................................................................... 7 VI. Jízdní doklady ........................................................................................................................ 8 1. Jednotlivé kilometrické jízdné ................................................................................................. 8 2. Dlouhodobé časové kilometrické jízdné (pouze v železniční dopravě) ................................... 9 3. Další jízdní doklady ................................................................................................................ 10 VII. Dovozné ............................................................................................................................. -

Eestimaa Looduse Fond Vilsandi Rahvuspargi Kaitsekorralduskava
ELF-i poolt Keskkonnaametile üle antud kinnitamata versioon Eestimaa Looduse Fond Vilsandi rahvuspargi kaitsekorralduskava aastateks 2011-2020 Liis Kuresoo ja Kaupo Kohv Tartu-Vilsandi 2010 ELF-i poolt Keskkonnaametile üle antud kinnitamata versioon SISUKORD Sissejuhatus ..................................................................................................................................... 6 1 Vilsandi rahvuspargi iseloomustus ......................................................................................... 8 1.1 Vilsandi rahvuspargi asend .......................................................................................... 8 1.2 Vilsandi rahvuspargi geomorfoloogiline ja bioloogiline iseloomustus ....................... 8 1.3 Vilsandi rahvuspargi kaitse-eesmärk, kaitsekord ja rahvusvaheline staatus................ 8 1.4 Maakasutus ja maaomand ............................................................................................ 9 1.5 Huvigrupid ................................................................................................................. 13 1.6 Vilsandi rahvuspargi visioon ..................................................................................... 16 2 Väärtused ja kaitse-eesmärgid .............................................................................................. 17 Elustik ........................................................................................................................................... 17 2.1 Linnustik ...................................................................................................................