Phytochemical Analysis of Symphytum Officinale Root Culture Extract
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Vascular Plants of Massachusetts
The Vascular Plants of Massachusetts: The Vascular Plants of Massachusetts: A County Checklist • First Revision Melissa Dow Cullina, Bryan Connolly, Bruce Sorrie and Paul Somers Somers Bruce Sorrie and Paul Connolly, Bryan Cullina, Melissa Dow Revision • First A County Checklist Plants of Massachusetts: Vascular The A County Checklist First Revision Melissa Dow Cullina, Bryan Connolly, Bruce Sorrie and Paul Somers Massachusetts Natural Heritage & Endangered Species Program Massachusetts Division of Fisheries and Wildlife Natural Heritage & Endangered Species Program The Natural Heritage & Endangered Species Program (NHESP), part of the Massachusetts Division of Fisheries and Wildlife, is one of the programs forming the Natural Heritage network. NHESP is responsible for the conservation and protection of hundreds of species that are not hunted, fished, trapped, or commercially harvested in the state. The Program's highest priority is protecting the 176 species of vertebrate and invertebrate animals and 259 species of native plants that are officially listed as Endangered, Threatened or of Special Concern in Massachusetts. Endangered species conservation in Massachusetts depends on you! A major source of funding for the protection of rare and endangered species comes from voluntary donations on state income tax forms. Contributions go to the Natural Heritage & Endangered Species Fund, which provides a portion of the operating budget for the Natural Heritage & Endangered Species Program. NHESP protects rare species through biological inventory, -

State of New York City's Plants 2018
STATE OF NEW YORK CITY’S PLANTS 2018 Daniel Atha & Brian Boom © 2018 The New York Botanical Garden All rights reserved ISBN 978-0-89327-955-4 Center for Conservation Strategy The New York Botanical Garden 2900 Southern Boulevard Bronx, NY 10458 All photos NYBG staff Citation: Atha, D. and B. Boom. 2018. State of New York City’s Plants 2018. Center for Conservation Strategy. The New York Botanical Garden, Bronx, NY. 132 pp. STATE OF NEW YORK CITY’S PLANTS 2018 4 EXECUTIVE SUMMARY 6 INTRODUCTION 10 DOCUMENTING THE CITY’S PLANTS 10 The Flora of New York City 11 Rare Species 14 Focus on Specific Area 16 Botanical Spectacle: Summer Snow 18 CITIZEN SCIENCE 20 THREATS TO THE CITY’S PLANTS 24 NEW YORK STATE PROHIBITED AND REGULATED INVASIVE SPECIES FOUND IN NEW YORK CITY 26 LOOKING AHEAD 27 CONTRIBUTORS AND ACKNOWLEGMENTS 30 LITERATURE CITED 31 APPENDIX Checklist of the Spontaneous Vascular Plants of New York City 32 Ferns and Fern Allies 35 Gymnosperms 36 Nymphaeales and Magnoliids 37 Monocots 67 Dicots 3 EXECUTIVE SUMMARY This report, State of New York City’s Plants 2018, is the first rankings of rare, threatened, endangered, and extinct species of what is envisioned by the Center for Conservation Strategy known from New York City, and based on this compilation of The New York Botanical Garden as annual updates thirteen percent of the City’s flora is imperiled or extinct in New summarizing the status of the spontaneous plant species of the York City. five boroughs of New York City. This year’s report deals with the City’s vascular plants (ferns and fern allies, gymnosperms, We have begun the process of assessing conservation status and flowering plants), but in the future it is planned to phase in at the local level for all species. -

FDA OTC Reviews Summary of Back Issues
Number 23 The Journal of the AMERICAN BOTANI CAL COUNCIL and the HERB RESEARCH FOUNDATION Chinese Medicinals -A Comprehensive Review of Chinese Materia Medica Legal and Regulatory- FDA OTC Reviews Summary of Back Issues Ongoing Market Report, Research Reviews (glimpses of studies published in over a dozen scientific and technical journals), Access, Book Reviews, Calendar, Legal and Regulatory, Herb Blurbs and Potpourri columns. #1 -Summer 83 (4 pp.) Eucalyptus Repels Reas, Stones Koalas; FDA OTC tiveness; Fungal Studies; More Polysaccharides; Recent Research on Ginseng; Heart Panel Reviews Menstrual & Aphrodisiac Herbs; Tabasco Toxicity?; Garlic Odor Peppers; Yew Continues to Amaze; Licorice O.D. Prevention; Ginseng in Perspec Repels Deer; and more. tive; Poisonous Plants Update; Medicinal Plant Conservation Project; 1989 Oberly #2- Fall/Winter 83-84 (8 pp.) Appeals Court Overrules FDA on Food Safety; Award Nominations; Trends in Self-Care Conference; License Plates to Fund Native FDA Magazine Pans Herbs; Beware of Bay Leaves; Tiny Tree: Cancer Cure?; Plant Manual; and more. Comfrey Tea Recall; plus. #17-Summer 88. (24 pp.) Sarsaparilla, A Literature Review by Christopher #3-Spring 84 (8 pp.) Celestial Sells to Kraft; Rowers and Dinosaurs Demise?; Hobbs; Hops May Help Metabolize Toxins; Herbal Roach Killer; Epazote Getting Citrus Peels for Kitty Litter; Saffron; Antibacterial Sassafras; WHO Studies Anti· More Popular, Aloe Market Levels Off; Herbal Tick Repellent?; Chinese Herb fertility Plants; Chinese Herbal Drugs; Feverfew Migraines; -

Conserving Europe's Threatened Plants
Conserving Europe’s threatened plants Progress towards Target 8 of the Global Strategy for Plant Conservation Conserving Europe’s threatened plants Progress towards Target 8 of the Global Strategy for Plant Conservation By Suzanne Sharrock and Meirion Jones May 2009 Recommended citation: Sharrock, S. and Jones, M., 2009. Conserving Europe’s threatened plants: Progress towards Target 8 of the Global Strategy for Plant Conservation Botanic Gardens Conservation International, Richmond, UK ISBN 978-1-905164-30-1 Published by Botanic Gardens Conservation International Descanso House, 199 Kew Road, Richmond, Surrey, TW9 3BW, UK Design: John Morgan, [email protected] Acknowledgements The work of establishing a consolidated list of threatened Photo credits European plants was first initiated by Hugh Synge who developed the original database on which this report is based. All images are credited to BGCI with the exceptions of: We are most grateful to Hugh for providing this database to page 5, Nikos Krigas; page 8. Christophe Libert; page 10, BGCI and advising on further development of the list. The Pawel Kos; page 12 (upper), Nikos Krigas; page 14: James exacting task of inputting data from national Red Lists was Hitchmough; page 16 (lower), Jože Bavcon; page 17 (upper), carried out by Chris Cockel and without his dedicated work, the Nkos Krigas; page 20 (upper), Anca Sarbu; page 21, Nikos list would not have been completed. Thank you for your efforts Krigas; page 22 (upper) Simon Williams; page 22 (lower), RBG Chris. We are grateful to all the members of the European Kew; page 23 (upper), Jo Packet; page 23 (lower), Sandrine Botanic Gardens Consortium and other colleagues from Europe Godefroid; page 24 (upper) Jože Bavcon; page 24 (lower), Frank who provided essential advice, guidance and supplementary Scumacher; page 25 (upper) Michael Burkart; page 25, (lower) information on the species included in the database. -

Diplomová Práce
Jihočeská univerzita v Českých Budějovicích Zemědělská fakulta Studijní program: N4101 Zemědělské inženýrství Studijní obor: Agroekologie Katedra: Rostlinné výroby a agroekologie Vedoucí katedry: prof. Ing. Vladislav Čurn, Ph.D. DIPLOMOVÁ PRÁCE Biologie, semenářské vlastnosti a zakládání porostů kostivalu lékařského (Symphytum officinale L.) Vedoucí diplomové práce: Ing. Milan Kobes, Ph.D. Autor diplomové práce: Bc. Jaroslava Kovářová České Budějovice, duben 2016 Čestné prohlášení Prohlašuji, že svou diplomovou práci s názvem „Biologie, semenářské vlastnosti a zakládání porostů kostivalu lékařského (Symphytum officinale L.)“ jsem vypracovala samostatně pod vedením vedoucí diplomové práce Ing. Milana Kobese, Ph.D a s použitím odborné literatury a dalších informačních zdrojů, které jsou v práci citovány a zároveň uvedeny v seznamu literatury na konci této práce. Jako autorka uvedené diplomové práce dále prohlašuji, že jsem v souvislosti s jejím vytvořením neporušila autorská práva třetích osob. Dále prohlašuji, že v souladu s § 47b zákona č. 111/1998 Sb. v platném znění souhlasím se zveřejněním své diplomové práce, a to v nezkrácené podobě (v úpravě vzniklé vypuštěním vyznačených částí archivovaných Zemědělskou fakultou JU) elektronickou cestou ve veřejně přístupné části databáze STAG provozované Jihočeskou univerzitou v Českých Budějovicích na jejích internetových stránkách. V Českých Budějovicích dne.......... _____________________________ Poděkování Velké poděkování patří mému vedoucímu práce Ing. Milanu Kobesovi, Ph.D. za odborné rady, připomínky, za vedení a odborné konzultace, které mi poskytl během zpracování této diplomové práce. Děkuji celé své rodině za podporu. ABSTRAKT Diplomová práce s názvem ,,Biologie, semenářské vlastnosti a zakládání porostů kostivalu lékařského (Symphytum officinale L.)“ se zabývala hodnocením způsobů rozmnožování kostivalu a hodnocením vlivu ekologických podmínek na růst kostivalu. Dále testy klíčivosti a jejich statistickým hodnocením při respektování specifik práce se semeny. -

Selection of Borage (Borago Officinalis) As a Seed Crop for Pharmaceutical Uses
Heredity 65 (1990) 249-257 The Genetical Society of Great Britain Received 8 March 1990 Selection of borage (Borago officinalis) as a seed crop for pharmaceutical uses N. W. Gaiwey and Department of Genetics, University of Cambridge, A. J. Shirlin Downing Street, Cambridge CB2 3EH, U.K. Gamma-linolenic acid (GLA) has been reported to be beneficial in the treatment of a wide range of disorders. It is extracted from the seed of the evening primrose, but the seed of borage is an alternative source of supply with agronomic advantages. Lines of borage of diverse origin were assembled, and selections for increased seed production, oil content and GLA content, and for reduced erucic acid content, were made. All these characteristics except for oil content were clearly heritable, but GLA content was negatively correlated with oil content and positively correlated with erucic acid content. Blue-flowered northern European genotypes had a higher GLA content than white-flowered cultivated genotypes from Spain. INTRODUCTION extraction easier. However, borage oil has the dis- advantage of containing 1-2 per cent of erucic Gamma-linolenicacid (GLA), taken orally, has acid. This compound, although it is an essential been reported to be beneficial in the treatment of fatty acid, is believed to be harmful in large doses, mild hypertension, elevated cholesterol levels, and breeders of oilseed rape have selected for low premenstrual syndrome, atopic eczema and other erucic acid content. disorders (Horrobin, 1984). These effects are prob- Borage has not been subjected to selection for ably related to the role of dietary essential fatty cultivation as a seed crop, and research was there- acids in determining the properties of membranes fore carried out with the aim of developing lines (Horrobin and Manku, 1983) and as precursors of that would be superior to those currently available prostaglandins, leukotrienes and related sub- as a source of GLA. -
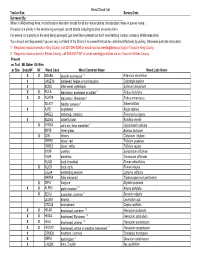
Weed Check List Survey Date
Weed Check List Trail or Site: Survey Date: Surveyed By: When in Wildnerness Area, record location and other details for all non-native plants. Include plant name in survey notes. If a weed is a priority in the area being surveyed, record details including location on survey form. For weeds not a priority in the area being surveyed, just mark them present but don't need lat/long location unless in Wilderness Area. You can pull and bag weeds if you are very confident of the ID and it is a weed that can be controlled effectively by pulling. Otherwise just note its location. 1 - Regulated noxious weeds in King County, call 206-296-0290 or email [email protected] if found in King County 2 - Regulated noxious weed in Kittitas County, call 509-962-7007 or email [email protected] if found in Kittitas County Present on Trail Mt. Baker Ok Wen or Site Snoq NF NF Weed Code Weed Common Name Weed Latin Name XXARAB3 absinth wormwood 1,2 Artemisia absinthium X CASE13 bindweed, hedge or morning glory Calystegia sepium X SODU bittersweet nightshade Solanum dulcamara XXRULA blackberry, evergreen or cutleaf 2 Rubus laciniatus XXRUAR9 blackberry, Himalayan 2 Rubus armeniacus SILA21 bladder campion 2 Silene latifolia X AJRE bugleweed Ajuga reptans RARE3 buttercup, creeping Ranunculus repens X BUDA2 butterfly bush Buddleia davidii X HYRA3 cat’s ear, false dandelion 2 Hypochaeris radicata BRTE cheat grass Bromus tectorum X CIIN chicory Cichorium intybus TRPR2 clover, red Trifolium pratense TRRE3 clover, white Trifolium repens SYOF comfrey Symphytum officinale TAOF dandelion Taraxacum officinale RUOB dock, broadleaf Rumex obtusifolius X RUCR dock, curly Rumex crispus LALA4 everlasting peavine Lathyrus latifolius MAPE2 false mayweed Tripleurospermum perforatum X DIPU foxglove Digitalis purpurea XXALPE4 garlic mustard 1,2 Alliaria petiolata X SEVU groundsel, common 2 Senecio vulgaris LEONT hawkbit Leontodon spp. -

Sterol Addition During Pollen Collection by Bees
Sterol addition during pollen collection by bees: another possible strategy to balance nutrient deficiencies? Maryse Vanderplanck, Pierre-Laurent Zerck, Georges Lognay, Denis Michez To cite this version: Maryse Vanderplanck, Pierre-Laurent Zerck, Georges Lognay, Denis Michez. Sterol addition during pollen collection by bees: another possible strategy to balance nutrient deficiencies?. Apidologie, 2020, 51 (5), pp.826-843. 10.1007/s13592-020-00764-3. hal-02784696 HAL Id: hal-02784696 https://hal.archives-ouvertes.fr/hal-02784696 Submitted on 3 May 2021 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Apidologie (2020) 51:826–843 Original article * INRAE, DIB and Springer-Verlag France SAS, part of Springer Nature, 2020 DOI: 10.1007/s13592-020-00764-3 Sterol addition during pollen collection by bees: another possible strategy to balance nutrient deficiencies? 1,2 1 3 1 Maryse VANDERPLANCK , Pierre-Laurent ZERCK , Georges LOGNAY , Denis MICHEZ 1Laboratory of Zoology, Research Institute for Biosciences, University of Mons, 20 Place du Parc, 7000, Mons, Belgium 2CNRS, UMR 8198 - Evo-Eco-Paleo, Univ. Lille, F-59000, Lille, France 3Analytical Chemistry, Agro Bio Chem Department, Gembloux Agro-Bio Tech University of Liège, 2 Passage des Déportés, 5030, Gembloux, Belgium Received 10 July 2019 – Revised2March2020– Accepted 30 March 2020 Abstract – Sterols are essential nutrients for bees which are thought to obtain them exclusively from pollen. -

Draft Assessment Report on Symphytum Officinale L., Radix
12 July 2011 EMA/HMPC/572844/2009 Committee on Herbal Medicinal Products (HMPC) Assessment report on Symphytum officinale L., radix Based on Article 16d(1), Article 16f and Article 16h of Directive 2001/83/EC as amended (traditional use) Draft Herbal substance(s) (binomial scientific name of the plant, including plant part) Symphytum officinale L., radix Herbal preparation(s) Liquid extract (DER 2:1), extraction solvent 65% (V/V) ethanol Pharmaceutical forms Herbal preparations in semi-solid dosage forms for cutaneous use Rapporteur Assessor(s) Note: This Assessment Report is published to support the release for public consultation of the draft Community herbal monograph on Symphytum officinale L., radix. It should be noted that this document is a working document, not yet fully edited, and which shall be further developed after the release for consultation of the monograph. Interested parties are welcome to submit comments to the HMPC secretariat, which the Rapporteur and the MLWP will take into consideration but no ‘overview of comments received during the public consultation’ will be prepared in relation to the comments that will be received on this assessment report. The publication of this draft assessment report has been agreed to facilitate the understanding by Interested Parties of the assessment that has been carried out so far and led to the preparation of the draft monograph. 7 Westferry Circus ● Canary Wharf ● London E14 4HB ● United Kingdom Telephone +44 (0)20 7418 8400 Facsimile +44 (0)20 7523 7051 E-mail [email protected] Website www.ema.europa.eu An agency of the European Union © European Medicines Agency, 2011. -

Public Statement on the Use of Herbal Medicinal Products Containing Pyrrolizidine Alkaloids (Pas) EMA/HMPC/893108/2011 Page 2/22 30 1
1 06 November 2013 2 EMA/HMPC/893108/2011 3 Committee on Herbal Medicinal Products (HMPC) 4 Public statement on the use of herbal medicinal products 5 containing toxic, unsaturated pyrrolizidine alkaloids (PAs) 6 7 2nd Draft 8 Draft discussed by Working Party on Community monographs and November 2011 Community list (MLWP) January 2012 March 2012 May 2012 Adopted by Committee on Herbal Medicinal Products (HMPC) for release 24 September 2012 for consultation Start of public consultation 25 October 2012 End of consultation (deadline for comments) 15 February 2013 2nd draft agreed by Working Party on Community monographs and July 2013 Community list (MLWP) Coordination with Safety Working Party July - October 2013 2nd draft adopted by Committee on Herbal Medicinal Products (HMPC) 17 September 2013 Start of public consultation 14 November 2013 End of consultation (deadline for comments) 15 February 2014 9 Comments should be provided using this template. The completed comments form should be sent to [email protected] 10 Keywords Herbal medicinal products; HMPC; pyrrolizidine alkaloids 11 7 Westferry Circus ● Canary Wharf ● London E14 4HB ● United Kingdom Telephone +44 (0)20 7418 8400 Facsimile +44 (0)20 7523 7051 E-mail [email protected] Website www.ema.europa.eu An agency of the European Union © European Medicines Agency, 2013. Reproduction is authorised provided the source is acknowledged. 12 Public statement on the use of herbal medicinal products 13 containing toxic, unsaturated pyrrolizidine alkaloids (PAs) 14 15 Table of contents 16 1. Introduction (Problem statement) .......................................................... 3 17 1.1. Pyrrolizidine alkaloids (PAs) ...................................................................................3 18 1.2. -

The Chemical Composition, Botanical Characteristic and Biological Activities of Borago Officinalis: a Review
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Asian Pac J Trop Med 2014; 7(Suppl 1):: S22-S28 S22 Contents lists available at ScienceDirect Asian Pacific Journal of Tropical Medicine journal homepage:www.elsevier.com/locate/apjtm Document heading doi: 10.1016/S1995-7645(14)60199-1 The chemical composition, botanical characteristic and biological activities of Borago officinalis: a review 1 2 1 Majid Asadi-Samani , Mahmoud Bahmani , Mahmoud Rafieian-Kopaei * 1Medical Plants Research Center, Shahrekord University of Medical Sciences, Shahrekord, Iran 2Razi Herbal Medicines Research Center, Lorestan University of Medical Sciences, Khorramabad, Iran ARTICLE INFO ABSTRACT Article history: Borago officinalis Borage ( ) is an annual herb which is cultivated for medicinal and culinary uses, Received 2 Jun 2014 although it is commercially cultivated for borage seed oil. Borage seed oil is the plant rich in the Received in revised form 23 Aug 2014 gamma-linolenic acid (26%-38%) which is used as dietary or food supplement. Other than seed oil Accepted 17 Sep 2014 it contains a lot of fatty acids such as linoleic acid (35%-38%), oleic acid (16%-20%), palmitic acid Available online 26 Sep 2014 (10%-11%), stearic acid (3.5%-4.5%), eicosenoic acid (3.5%-5.5%) and erucic acid (1.5%-3.5%). It is used for the treatment of various diseases such as multiple sclerosis, diabetes, heart diseases, arthritis and eczema. In this study different aspects of borage such as plant characteristics,’ production, applications in traditional medicine, clinical considerations, its effects on patients Keywords: blood and urine biochemistry, and also the effect of the its products on liver and kidney Borage performance tests are presented using published articles in scientific sites. -

Comfrey (Symphytum Spp.) As an Alternative field Crop Contributing to Closed Agricultural Cycles in Chicken Feeding
Science of the Total Environment 742 (2020) 140490 Contents lists available at ScienceDirect Science of the Total Environment journal homepage: www.elsevier.com/locate/scitotenv Comfrey (Symphytum spp.) as an alternative field crop contributing to closed agricultural cycles in chicken feeding Michael Oster a, Henry Reyer a, Jonas Keiler b, Elizabeth Ball c, Christina Mulvenna c, Eduard Muráni a, Siriluck Ponsuksili a, Klaus Wimmers a,d,⁎ a Leibniz Institute for Farm Animal Biology (FBN), Wilhelm-Stahl-Allee 2, 18196 Dummerstorf, Germany b Institute of Anatomy, Rostock University Medical Center, Gertrudenstrasse 9, 18057 Rostock, Germany c Agri-Food and Biosciences Institute, Large Park, Hillsborough, Co Down BT26 6DR, UK d Faculty of Agricultural and Environmental Sciences, University Rostock, 18059 Rostock, Germany HIGHLIGHTS GRAPHICAL ABSTRACT • Comfrey as a regionally adapted feed crop could be used in poultry farming. • Intake of comfrey leaves showed no ab- erration of health and tissue integrity. • Comfrey leaves in poultry feed can ef- fectively replace rock mineral supple- ments. • Close nutrient cycles by reactivating phosphorus from over-fertilized soils article info abstract Article history: Local cultivars of comfrey (Symphytum spp.) have been used to cover protein and mineral requirements of farm Received 22 April 2020 animals in low-input systems. Due to its known health-promoting (e.g. allantoin), but also anti-nutritive ingre- Received in revised form 21 June 2020 dients (e.g. pyrrolizidine alkaloids), multidisciplinary approaches are essential in order to quantify the nutritional Accepted 22 June 2020 value and the potential of its use in poultry and farm animals in terms of meeting animal needs, using local re- Available online 27 June 2020 sources as well as remediating over-fertilized soils.