Apr 2 4 1996
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Dual Language of Geometry in Gothic Architecture: the Symbolic Message of Euclidian Geometry Versus the Visual Dialogue of Fractal Geometry
Peregrinations: Journal of Medieval Art and Architecture Volume 5 Issue 2 135-172 2015 The Dual Language of Geometry in Gothic Architecture: The Symbolic Message of Euclidian Geometry versus the Visual Dialogue of Fractal Geometry Nelly Shafik Ramzy Sinai University Follow this and additional works at: https://digital.kenyon.edu/perejournal Part of the Ancient, Medieval, Renaissance and Baroque Art and Architecture Commons Recommended Citation Ramzy, Nelly Shafik. "The Dual Language of Geometry in Gothic Architecture: The Symbolic Message of Euclidian Geometry versus the Visual Dialogue of Fractal Geometry." Peregrinations: Journal of Medieval Art and Architecture 5, 2 (2015): 135-172. https://digital.kenyon.edu/perejournal/vol5/iss2/7 This Feature Article is brought to you for free and open access by the Art History at Digital Kenyon: Research, Scholarship, and Creative Exchange. It has been accepted for inclusion in Peregrinations: Journal of Medieval Art and Architecture by an authorized editor of Digital Kenyon: Research, Scholarship, and Creative Exchange. For more information, please contact [email protected]. Ramzy The Dual Language of Geometry in Gothic Architecture: The Symbolic Message of Euclidian Geometry versus the Visual Dialogue of Fractal Geometry By Nelly Shafik Ramzy, Department of Architectural Engineering, Faculty of Engineering Sciences, Sinai University, El Masaeed, El Arish City, Egypt 1. Introduction When performing geometrical analysis of historical buildings, it is important to keep in mind what were the intentions -

The Solutions to Uncertainty Problem of Urban Fractal Dimension Calculation
The Solutions to Uncertainty Problem of Urban Fractal Dimension Calculation Yanguang Chen (Department of Geography, College of Urban and Environmental Sciences, Peking University, Beijing 100871, P.R. China. E-mail: [email protected]) Abstract: Fractal geometry provides a powerful tool for scale-free spatial analysis of cities, but the fractal dimension calculation results always depend on methods and scopes of study area. This phenomenon has been puzzling many researchers. This paper is devoted to discussing the problem of uncertainty of fractal dimension estimation and the potential solutions to it. Using regular fractals as archetypes, we can reveal the causes and effects of the diversity of fractal dimension estimation results by analogy. The main factors influencing fractal dimension values of cities include prefractal structure, multi-scaling fractal patterns, and self-affine fractal growth. The solution to the problem is to substitute the real fractal dimension values with comparable fractal dimensions. The main measures are as follows: First, select a proper method for a special fractal study. Second, define a proper study area for a city according to a study aim, or define comparable study areas for different cities. These suggestions may be helpful for the students who takes interest in or even have already participated in the studies of fractal cities. Key words: Fractal; prefractal; multifractals; self-affine fractals; fractal cities; fractal dimension measurement 1. Introduction A scientific research is involved with two processes: description and understanding. A study often proceeds first by describing how a system works and then by understanding why (Gordon, 2005). Scientific description relies heavily on measurement and mathematical modeling, and scientific 1 explanation is mainly to use observation, experience, and experiment (Henry, 2002). -

Art and Engineering Inspired by Swarm Robotics
RICE UNIVERSITY Art and Engineering Inspired by Swarm Robotics by Yu Zhou A Thesis Submitted in Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy Approved, Thesis Committee: Ronald Goldman, Chair Professor of Computer Science Joe Warren Professor of Computer Science Marcia O'Malley Professor of Mechanical Engineering Houston, Texas April, 2017 ABSTRACT Art and Engineering Inspired by Swarm Robotics by Yu Zhou Swarm robotics has the potential to combine the power of the hive with the sen- sibility of the individual to solve non-traditional problems in mechanical, industrial, and architectural engineering and to develop exquisite art beyond the ken of most contemporary painters, sculptors, and architects. The goal of this thesis is to apply swarm robotics to the sublime and the quotidian to achieve this synergy between art and engineering. The potential applications of collective behaviors, manipulation, and self-assembly are quite extensive. We will concentrate our research on three topics: fractals, stabil- ity analysis, and building an enhanced multi-robot simulator. Self-assembly of swarm robots into fractal shapes can be used both for artistic purposes (fractal sculptures) and in engineering applications (fractal antennas). Stability analysis studies whether distributed swarm algorithms are stable and robust either to sensing or to numerical errors, and tries to provide solutions to avoid unstable robot configurations. Our enhanced multi-robot simulator supports this research by providing real-time simula- tions with customized parameters, and can become as well a platform for educating a new generation of artists and engineers. The goal of this thesis is to use techniques inspired by swarm robotics to develop a computational framework accessible to and suitable for both artists and engineers. -

Consensus and Coherence in Fractal Networks Stacy Patterson, Member, IEEE and Bassam Bamieh, Fellow, IEEE
1 Consensus and Coherence in Fractal Networks Stacy Patterson, Member, IEEE and Bassam Bamieh, Fellow, IEEE Abstract—We consider first and second order consensus algo- determined by the spectrum of the Laplacian, though in a rithms in networks with stochastic disturbances. We quantify the slightly more complex way [5]. deviation from consensus using the notion of network coherence, Several recent works have studied the relationship between which can be expressed as an H2 norm of the stochastic system. We use the setting of fractal networks to investigate the question network coherence and topology in first-order consensus sys- of whether a purely topological measure, such as the fractal tems. Young et al. [4] derived analytical expressions for dimension, can capture the asymptotics of coherence in the large coherence in rings, path graphs, and star graphs, and Zelazo system size limit. Our analysis for first-order systems is facilitated and Mesbahi [11] presented an analysis of network coherence by connections between first-order stochastic consensus and the in terms of the number of cycles in the graph. Our earlier global mean first passage time of random walks. We then show how to apply similar techniques to analyze second-order work [5] presented an asymptotic analysis of network coher- stochastic consensus systems. Our analysis reveals that two ence for both first and second order consensus algorithms in networks with the same fractal dimension can exhibit different torus and lattice networks in terms of the number of nodes asymptotic scalings for network coherence. Thus, this topological and the network dimension. These results show that there characterization of the network does not uniquely determine is a marked difference in coherence between first-order and coherence behavior. -

Structural Properties of Vicsek-Like Deterministic Multifractals
S S symmetry Article Structural Properties of Vicsek-like Deterministic Multifractals Eugen Mircea Anitas 1,2,* , Giorgia Marcelli 3 , Zsolt Szakacs 4 , Radu Todoran 4 and Daniela Todoran 4 1 Bogoliubov Laboratory of Theoretical Physics, Joint Institute for Nuclear Research, 141980 Dubna, Russian 2 Horia Hulubei, National Institute of Physics and Nuclear Engineering, 77125 Magurele, Romania 3 Faculty of Mathematical, Physical and Natural Sciences, Sapienza University of Rome, 00185 Rome, Italy; [email protected] 4 Faculty of Science, Technical University of Cluj Napoca, North University Center of Baia Mare, Baia Mare, 430122 Maramures, Romania; [email protected] (Z.S.); [email protected] (R.T.); [email protected] (D.T.) * Correspondence: [email protected] Received: 13 May 2019; Accepted: 15 June 2019; Published: 18 June 2019 Abstract: Deterministic nano-fractal structures have recently emerged, displaying huge potential for the fabrication of complex materials with predefined physical properties and functionalities. Exploiting the structural properties of fractals, such as symmetry and self-similarity, could greatly extend the applicability of such materials. Analyses of small-angle scattering (SAS) curves from deterministic fractal models with a single scaling factor have allowed the obtaining of valuable fractal properties but they are insufficient to describe non-uniform structures with rich scaling properties such as fractals with multiple scaling factors. To extract additional information about this class of fractal structures we performed an analysis of multifractal spectra and SAS intensity of a representative fractal model with two scaling factors—termed Vicsek-like fractal. We observed that the box-counting fractal dimension in multifractal spectra coincide with the scattering exponent of SAS curves in mass-fractal regions. -

Application of Fractal Geometry PLANNING Built Environment
M. Jevrić i dr. Primjena fraktalne geometrije u projektiranju gradskog obrasca ISSN 1330-3651 (Print), ISSN 1848-6339 (Online) UDC/UDK 514.12:711.4.013 APPLICATION OF FRACTAL GEOMETRY IN URBAN PATTERN DESIGN Marija Jevrić, Miloš Knežević, Jelisava Kalezić, Nataša Kopitović-Vuković, Ivana Ćipranić Preliminary notes Fractal geometry studies the structures characterized by the repetition of the same principles of element distribution on multiple levels of observation, which is recognized in the case of cities. Since the holistic approach to planning involves the introduction of new processes and procedures regarding the consideration of all relevant factors that can influence the choice of urban pattern solutions, we point to the awareness of the importance of fractality in natural and built environment for human well-being. In this paper, we have reminded of the fractality of urban environment as a quality of an urban area and pointed on the disadvantages of conventional planning concepts, which do not consider pattern fractality as criterion for environmental improvement. After a brief review of the basis of fractal theory and the review of previous studies, we have presented previously known and suggested new possibilities for the application of fractal geometry in urban pattern design. Keywords: fractal geometry, fractals, holistic approach, urban pattern design Primjena fraktalne geometrije u projektiranju gradskog obrasca Prethodno priopćenje Fraktalna geometrija proučava objekte koje karakterizira ponavljanje istih principa raspodjele elemenata promatranjem na više nivoa, a to se prepoznaje u slučaju gradova. Budući da holistički pristup planiranju uključuje uvođenje novih procesa i postupaka u odnosu na razmatranje svih relevantnih faktora koji mogu utjecati na izbor rješenja gradskog obrasca, naglašavamo koliko je za ljudsko blagostanje potrebna svijest o važnosti fraktalnosti u prirodnom i izgrađenom okolišu. -

A Generalization of the Hausdorff Dimension Theorem for Deterministic Fractals
mathematics Article A Generalization of the Hausdorff Dimension Theorem for Deterministic Fractals Mohsen Soltanifar 1,2 1 Biostatistics Division, Dalla Lana School of Public Health, University of Toronto, 620-155 College Street, Toronto, ON M5T 3M7, Canada; [email protected] 2 Real World Analytics, Cytel Canada Health Inc., 802-777 West Broadway, Vancouver, BC V5Z 1J5, Canada Abstract: How many fractals exist in nature or the virtual world? In this paper, we partially answer the second question using Mandelbrot’s fundamental definition of fractals and their quantities of the Hausdorff dimension and Lebesgue measure. We prove the existence of aleph-two of virtual fractals with a Hausdorff dimension of a bi-variate function of them and the given Lebesgue measure. The question remains unanswered for other fractal dimensions. Keywords: cantor set; fractals; Hausdorff dimension; continuum; aleph-two MSC: 28A80; 03E10 1. Introduction Benoit Mandelbrot (1924–2010) coined the term fractal and its dimension in his 1975 essay on the quest to present a mathematical model for self-similar, fractured ge- ometric shapes in nature [1]. Both nature and the virtual world have prominent examples Citation: Soltanifar, M. A of fractals: some natural examples include snowflakes, clouds and mountain ranges, while Generalization of the Hausdorff some virtual instances are the middle third Cantor set, the Sierpinski triangle and the Dimension Theorem for Deterministic Vicsek fractal. A key element in the definition of the term fractal is its dimension usually Fractals. Mathematics 2021, 9, 1546. indexed by the Hausdorff dimension. This dimension has played vital roles in develop- https://doi.org/10.3390/math9131546 ing the concept of fractal dimension and the definition of the term fractal and has had extensive applications [2–5]. -

Topological Conditions on Inhomogeneous Fractals in Martin Boundary Theory and Their Algorithmic Testing
Topological conditions on inhomogeneous fractals in Martin boundary theory and their algorithmic testing Stefan Kohl∗ June 11, 2021 Abstract We want to consider Martin boundary theory applied to inhomogeneous fractals. This is under some conditions possible, but up to now it is not clear, how one can easily check, if a certain fractal fulfills those conditions. We want to simplify one condition and further develop a computer algorithm, which can check, if an attractor fulfills the condition. 2010 Mathematics Subject Classification: 28A80, 31C35, 60J10, 60J45 Keywords: Martin boundary theory, Markov chains, Green function, fractals, multifractals. 1 Introduction In a recent paper Freiberg and Kohl [FK19] studied the possibility to identify the attractor of a weighted iterated function system with the Martin boundary. To do so, they followed mainly the idea of Denker/Sato [DS01] and Lau/Ngai/Wang [LN12, LW15]. They adapted the transition probability of the associated Markov chain and were able to determine the Martin boundary under two conditions. The first condition, called (B1), is simple and easy to verify. The second condition (B2) is quite harder and it is not obvious, if a fractal fulfills (B2). Because of this we want to investigate (B2), determine some properties and make this condition easier to manage. The outline of this article is as follows: in section 2 we want to introduce the notation and summarize the work of [FK19]. We do not need all aspects of this article and thus we only present the most necessary. In section 3 we investigate (B2) and reduce it in the sense that we only have to consider a finite number of words instead of an infinite number as it is in the original work. -

Parallel Diffusion-Limited Aggregation
PHYSICAL REVIEW E VOLUME 52, NUMBER 5 NOVEMBER 1995 Parallel diffusion-limited aggregation Henry Kaufman,! Alessandro VespignanV'· Benoit B. Mandelbrot,!·2 and Lionel Woog! lDepartment of Mathematics, Yale University, New Haven, Connecticut 06520-8283 2Thomas J. Watson Research Center, Yorktown Heights, New York 10598-0218 (Received 21 March 1995) We present methods for simulating very large diffusion-limited aggregation (DLA) clusters using parallel processing (PDLA). With our techniques, we have been able to simulate clusters of up to 130 million particles. The time required for generating a 100 million particle PDLA is approximately 13 h. The fractal behavior of these "parallel" clusters changes from a multi particle aggregation dynamics to the usual DLA dynamics. The transition is described by simple scaling assumptions that define a charac teristic cluster size separating the two dynamical regimes. We also use DLA clusters as seeds for parallel processing. In this case, the transient regime disappears and the dynamics converges from the early stage to that of DLA. PACS number(s): 02.70.-c, 68.70.+w, 05.40.+j, 02.50.-r I. INTRODUCTION several quantities of the PDLA. From simple scaling as sumptions we can characterize the transient region and A model of irreversible growth to generate fractal find the behavior of the characteristic cluster size for structures was the diffusion limited aggregation (DLA) which the dynamics become identical to DLA's dynam model of Witten and Sander [1]. This model accounts for ics. the origin of fractal structures in a great variety of pro To avoid the effects of the dynamical drift and to speed cesses: dendritic growth, viscous fingers in fluids, dielec up the convergence towards the DLA regime we propose tric breakdown, electrochemical deposition, etc. -

An Interface Problem for a Sierpinski and a Vicsek Fractal
Math. Nachr. 280, No. 13–14, 1577 – 1594 (2007) / DOI 10.1002/mana.200410566 An interface problem for a Sierpinski and a Vicsek fractal Volker Metz ∗1 and Peter Grabner∗∗2 1 Bielefeld University, 33501 Bielefeld, Germany 2 Institute of Mathematics A, Graz University of Technology, Steyrergasse 30, 8010 Graz, Austria Received 29 December 2004, revised 28 October 2005, accepted 27 January 2006 Published online 6 September 2007 Key words Laplace operator, Brownian motion, interface, fractals MSC (2000) 31C25, 60J45, 80A28 We suggest a flexible way to study the self-similar interface of two different fractals. In contrast to previous methods the participating energies are modified in the neighborhood of the intersection of the fractals. In the example of the Vicsek snowflake and the 3-gasket, a variant of the Sierpinski gasket, we calculate the admissible transition constants via the “Short-cut Test”. The resulting range of values is reinterpreted in terms of traces of Lipschitz spaces on the intersection. This allows us to describe the interface effects of different transition constants and indicates the techniques necessary to generalize the present interface results. c 2007 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim 1 Introduction and results In 1993 Lindstrøm was the first to embed a fractal, the Sierpinski gasket, into the Euclidean plane and connect them [20]. Later on Kumagai, Jonsson and Hambly/Kumagai extended his work by analytical means [17, 12, 9]. They identified the domain of the fractal Dirichlet form as a certain Lipschitz space and used an extension map of Jonsson and Wallin, [14], to extend these functions to the plane. -
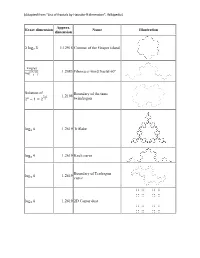
Fractal Examples Handout
(Adapted from “List of fractals by Hausdorff dimension”, Wikipedia) Approx. Exact dimension Name Illustration dimension 2 log 3 1.12915 Contour of the Gosper island 7 ( ) 1.2083 Fibonacci word fractal 60° 3 log φ 3+√13 log� 2 � Solution of Boundary of the tame 1.2108 2 1 = 2 twindragon 2− 2 − log 4 1.2619 Triflake 3 log 4 1.2619 Koch curve 3 Boundary of Terdragon log 4 1.2619 curve 3 log 4 1.2619 2D Cantor dust 3 (Adapted from “List of fractals by Hausdorff dimension”, Wikipedia) log 4 1.2619 2D L-system branch 3 log 5 1.4649 Vicsek fractal 3 Quadratic von Koch curve log 5 1.4649 (type 1) 3 log 1.4961 Quadric cross 10 √5 � 3 � Quadratic von Koch curve log 8 = 1.5000 3 (type 2) 4 2 log 3 1.5849 3-branches tree 2 (Adapted from “List of fractals by Hausdorff dimension”, Wikipedia) log 3 1.5849 Sierpinski triangle 2 log 3 1.5849 Sierpiński arrowhead curve 2 Boundary of the T-square log 3 1.5849 fractal 2 log = 1.61803 A golden dragon � 1 + log 2 1.6309 Pascal triangle modulo 3 3 1 + log 2 1.6309 Sierpinski Hexagon 3 3 log 1.6379 Fibonacci word fractal 1+√2 Solution of Attractor of IFS with 3 + + 1.6402 similarities of ratios 1/3, 1/2 1 1 and 2/3 3 = 12 � � � � 2 �3� (Adapted from “List of fractals by Hausdorff dimension”, Wikipedia) 32-segment quadric fractal log 32 = 1.6667 5 (1/8 scaling rule) 8 3 1 + log 3 1.6826 Pascal triangle modulo 5 5 50 segment quadric fractal 1 + log 5 1.6990 (1/10 scaling rule) 10 4 log 2 1.7227 Pinwheel fractal 5 log 7 1.7712 Hexaflake 3 ( ) 1.7848 Von Koch curve 85° log 4 log 2+cos 85° log 6 1.8617 Pentaflake -
![Arxiv:2008.12131V2 [Math.PR] 9 Nov 2020 1 Introduction](https://docslib.b-cdn.net/cover/9684/arxiv-2008-12131v2-math-pr-9-nov-2020-1-introduction-5429684.webp)
Arxiv:2008.12131V2 [Math.PR] 9 Nov 2020 1 Introduction
Exact mean first-passage time on generalized Vicsek fractal Fei Maa;1 Xiaomin Wanga Ping Wangb;c;d and Xudong Luoe a School of Electronics Engineering and Computer Science, Peking University, Beijing 100871, China b National Engineering Research Center for Software Engineering, Peking University, Beijing, China c School of Software and Microelectronics, Peking University, Beijing 102600, China d Key Laboratory of High Confidence Software Technologies (PKU), Ministry of Education, Beijing, China e College of Mathematics and Statistics, Northwest Normal University, Lanzhou 730070, China Abstract: Fractal phenomena may be widely observed in a great number of complex sys- tems. In this paper, we revisit the well-known Vicsek fractal, and study some of its structural properties for purpose of understanding how the underlying topology influences its dynamic behaviors. For instance, we analytically determine the exact solution to mean first-passage time for random walks on Vicsek fractal in a more light mapping-based manner than previous other methods, including typical spectral technique. More importantly, our method can be quite efficient to precisely calculate the solutions to mean first-passage time on all generalized versions of Vicsek fractal generated based on an arbitrary allowed seed, while other previous methods suitable for typical Vicsek fractal will become prohibitively complicated and even fail. Lastly, this analytic results suggest that the scaling relation between mean first-passage time and vertex number in generalized versions of Vicsek fractal keeps unchanged in the large graph size limit no matter what seed is selected. Keywords: Vicsek fractal, Random walks, Mean first-passage time. arXiv:2008.12131v2 [math.PR] 9 Nov 2020 1 Introduction Fractals are intriguing and may be popularly seen in a great deal of complex systems [1].