Chapter 4 Biological Environment
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pond-Breeding Amphibian Guild
Supplemental Volume: Species of Conservation Concern SC SWAP 2015 Pond-breeding Amphibians Guild Primary Species: Flatwoods Salamander Ambystoma cingulatum Carolina Gopher Frog Rana capito capito Broad-Striped Dwarf Siren Pseudobranchus striatus striatus Tiger Salamander Ambystoma tigrinum Secondary Species: Upland Chorus Frog Pseudacris feriarum -Coastal Plain only Northern Cricket Frog Acris crepitans -Coastal Plain only Contributors (2005): Stephen Bennett and Kurt A. Buhlmann [SCDNR] Reviewed and Edited (2012): Stephen Bennett (SCDNR), Kurt A. Buhlmann (SREL), and Jeff Camper (Francis Marion University) DESCRIPTION Taxonomy and Basic Descriptions This guild contains 4 primary species: the flatwoods salamander, Carolina gopher frog, dwarf siren, and tiger salamander; and 2 secondary species: upland chorus frog and northern cricket frog. Primary species are high priority species that are directly tied to a unifying feature or habitat. Secondary species are priority species that may occur in, or be related to, the unifying feature at some time in their life. The flatwoods salamander—in particular, the frosted flatwoods salamander— and tiger salamander are members of the family Ambystomatidae, the mole salamanders. Both species are large; the tiger salamander is the largest terrestrial salamander in the eastern United States. The Photo by SC DNR flatwoods salamander can reach lengths of 9 to 12 cm (3.5 to 4.7 in.) as an adult. This species is dark, ranging from black to dark brown with silver-white reticulated markings (Conant and Collins 1991; Martof et al. 1980). The tiger salamander can reach lengths of 18 to 20 cm (7.1 to 7.9 in.) as an adult; maximum size is approximately 30 cm (11.8 in.). -

Class: Amphibia Amphibians Order
CLASS: AMPHIBIA AMPHIBIANS ANNIELLIDAE (Legless Lizards & Allies) CLASS: AMPHIBIA AMPHIBIANS Anniella (Legless Lizards) ORDER: ANURA FROGS AND TOADS ___Silvery Legless Lizard .......................... DS,RI,UR – uD ORDER: ANURA FROGS AND TOADS BUFONIDAE (True Toad Family) BUFONIDAE (True Toad Family) ___Southern Alligator Lizard ............................ RI,DE – fD Bufo (True Toads) Suborder: SERPENTES SNAKES Bufo (True Toads) ___California (Western) Toad.............. AQ,DS,RI,UR – cN ___California (Western) Toad ............. AQ,DS,RI,UR – cN ANNIELLIDAE (Legless Lizards & Allies) Anniella ___Red-spotted Toad ...................................... AQ,DS - cN BOIDAE (Boas & Pythons) ___Red-spotted Toad ...................................... AQ,DS - cN (Legless Lizards) Charina (Rosy & Rubber Boas) ___Silvery Legless Lizard .......................... DS,RI,UR – uD HYLIDAE (Chorus Frog and Treefrog Family) ___Rosy Boa ............................................ DS,CH,RO – fN HYLIDAE (Chorus Frog and Treefrog Family) Pseudacris (Chorus Frogs) Pseudacris (Chorus Frogs) Suborder: SERPENTES SNAKES ___California Chorus Frog ............ AQ,DS,RI,DE,RO – cN COLUBRIDAE (Colubrid Snakes) ___California Chorus Frog ............ AQ,DS,RI,DE,RO – cN ___Pacific Chorus Frog ....................... AQ,DS,RI,DE – cN Arizona (Glossy Snakes) ___Pacific Chorus Frog ........................AQ,DS,RI,DE – cN BOIDAE (Boas & Pythons) ___Glossy Snake ........................................... DS,SA – cN Charina (Rosy & Rubber Boas) RANIDAE (True Frog Family) -

Frogs and Toads Defined
by Christopher A. Urban Chief, Natural Diversity Section Frogs and toads defined Frogs and toads are in the class Two of Pennsylvania’s most common toad and “Amphibia.” Amphibians have frog species are the eastern American toad backbones like mammals, but unlike mammals they cannot internally (Bufo americanus americanus) and the pickerel regulate their body temperature and frog (Rana palustris). These two species exemplify are therefore called “cold-blooded” (ectothermic) animals. This means the physical, behavioral, that the animal has to move ecological and habitat to warm or cool places to change its body tempera- similarities and ture to the appropriate differences in the comfort level. Another major difference frogs and toads of between amphibians and Pennsylvania. other animals is that amphibians can breathe through the skin on photo-Andrew L. Shiels L. photo-Andrew www.fish.state.pa.us Pennsylvania Angler & Boater • March-April 2005 15 land and absorb oxygen through the weeks in some species to 60 days in (plant-eating) beginning, they have skin while underwater. Unlike reptiles, others. Frogs can become fully now developed into insectivores amphibians lack claws and nails on their developed in 60 days, but many (insect-eaters). Then they leave the toes and fingers, and they have moist, species like the green frog and bullfrog water in search of food such as small permeable and glandular skin. Their can “overwinter” as tadpoles in the insects, spiders and other inverte- skin lacks scales or feathers. bottom of ponds and take up to two brates. Frogs and toads belong to the years to transform fully into adult Where they go in search of this amphibian order Anura. -

NMFS and USFWS Biological Assessment
LOS GATOS CREEK BRIDGE REPLACEMENT / SOUTH TERMINAL PHASE III PROJECT NMFS and USFWS Biological Assessment Prepared for Peninsula Corridor Joint Powers Board 1250 San Carlos Avenue P.O. Box 3006 San Carlos, California 94070-1306 and the Federal Transit Administration Region IX U.S. Department of Transportation 201 Mission Street Suite1650 San Francisco, CA 94105-1839 Prepared by HDR Engineering, Inc. 2379 Gateway Oaks Drive Suite 200 Sacramento, California 95833 August 2013 NMFS and USFWS Biological Assessment Prepared for Peninsula Corridor Joint Powers Board 1250 San Carlos Avenue P.O. Box 3006 San Carlos, California 94070-1306 and the Federal Transit Administration Region IX U.S. Department of Transportation 201 Mission Street Suite 1650 San Francisco, CA 94105-1839 Prepared by HDR Engineering, Inc. 2379 Gateway Oaks Drive, Suite 200 Sacramento, California 95833 August 2013 This page left blank intentionally. Summary The Peninsula Corridor Joint Powers Board (JPB) which operates the San Francisco Bay Area’s Caltrain passenger rail service proposes to replace the two-track railroad bridge that crosses Los Gatos Creek, in the City of San Jose, Santa Clara County, California. The Proposed Action is needed to address the structural deficiencies and safety issues of the Caltrain Los Gatos Creek railroad bridge to be consistent with the standards of safety and reliability required for public transit, to ensure that the bridge will continue to safely carry commuter rail service well into the future, and to improve operations at nearby San Jose Diridon Station and along the Caltrain rail line. This Biological Assessment (BA) has been prepared for the National Marine Fisheries Service (NMFS) and U.S. -

145-152 Zunika Amit.Pmd
Malays. Appl. Biol. (2018) 47(6): 145–152 ANTIMICROBIAL ACTIVITY OF PARTIALLY PURIFIED PEPTIDES ISOLATED FROM THE SKIN SECRETIONS OF BORNEAN FROGS IN THE FAMILY OF RANIDAE MUNA SABRI1,2, ELIZABETH JEGA JENGGUT1, RAMLAH ZAINUDIN3 and ZUNIKA AMIT1* 1Department of Basic Medical Sciences, Faculty of Medicine and Health Sciences, Universiti Malaysia Sarawak, 94300 Kota Samarahan, Sarawak, Malaysia 2Centre of PreUniversity Studies, Universiti Malaysia Sarawak, 94300 Kota Samarahan, Sarawak, Malaysia 3Department of Zoology, Faculty of Resource Science and Technology, Universiti Malaysia Sarawak, 94300 Kota Samarahan, Sarawak, Malaysia *E-mail: [email protected] Accepted 20 December 2018, Published online 31 December 2018 The emergence of drug resistant bacteria has now the head and neck of the frogs (Rollin-Smith et al., become a major public health problem worldwide 2002). Most AMPs are cationic in nature and share (Cohen, 2000; Kumarasamy et al., 2010; Sengupta a net positive charge at neutral pH with the high et al., 2013). WHO report (2017) on global content of hydrophobic residues and an amphipathic surveillance of antimicrobial resistance revealed a character (Galdiero et al., 2013; Power & Hancock, widespread development of resistance in both gram 2003). These characteristics allow the frog skin positive and gram negative bacteria which had peptides to kill bacteria through cell lysis by threatened millions of people worldwide. A rapid binding to negatively charged components of the increase in the number of drug-resistant bacteria bacterial membrane (Schadich et al., 2013). The and the incidence nosocomial infections pose a AMPs attract attention due to their effectiveness in challenge to conventional therapies using existing killing both gram-negative and gram-positive antibiotics, leading to the need in finding bacteria, without any of the undesirable effects of alternative microbicides to control these infections antibiotic resistance (Conlon and Sonnevand, 2011; (Lakshmaiah et al., 2015). -

Amphibious Arizona
AMPHIBIOUS ARIZONA Although Arizona is a pretty arid and dry state, Arizona is home to 26 different species of frogs and toads, 23 of which are considered indigenous, or native to the state. The Colorado River Toad Did you know? The State Amphibian is the Arizona Treefrog (Incilius alvarius) (Hyla wrightorum). The Colorado River Frogs have always been important to the Toad, also called the people of Arizona because the presence of frogs means that water is near. Many Sonoran Desert Toad, Native American people in Arizona use is a toad that is native frogs to symbolize water or rain and the to almost half of the sound of frogs signals monsoon season state of Arizona. These for Arizona. toads are among the All toads are frogs but not all frogs are toads. ©2006 Gary Nafis/ASDM Sonoran largest in the state, Frogs have smooth, slimy skin while toads Desert Digital Library look bumpy and drier. and only come out The Chiricahua Leopard Frog (Lithobates during the rainy season. They eat primarily beetles, chiricahuensis) is a threatened species. but are known to eat other insects and small They’re a “true frog,” which means that they vertebrates like other frogs and toads. The Colorado need access to water continuously. Livestock River Toad provides a lot of the music of summer grazing, urbanization, water diversion, and groundwater pumping all threaten the with their croaking, but they also make some people Chiricahua Leopard Frog. anxious: Colorado River Toads secrete a poison called a Think about it! bufotoxin, which is How do you think the growth of cities have highly toxic to cats impacted Arizona’s amphibians? and dogs. -
C:\Program Files\Adobe\Acrobat 4.0\Acrobat\Plug Ins\Openall
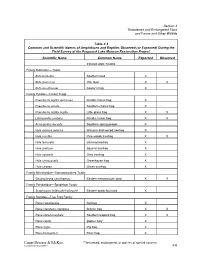
Section 4 Threatened and Endangered Flora and Fauna and Other Wildlife Table 4-3 Common and Scientific Names of Amphibians and Reptiles Observed (or Expected) During the Field Survey of the Proposed Lake Munson Restoration Project Scientific Name Common Name Expected Observed FROGS AND TOADS Family Bufonidae—Toads Bufo terrestris Southern toad X Bufo quercicus Oak toad X X Bufo woodhousei Fowler’s frog X Family Hylidae—Cricket Frogs Pseudacris nigrita verrucosa Florida chorus frog X Pseudacris ornata Southern chorus frog X Pseudacris nigrita nigrita Little grass frog X X Limnaoedus ocularis Florida cricket frog X X Acris gryllus dorsalis Southern spring peeper X Hyla avivoca avivoca Western bird-voiced treefrog X Hyla crucifer Pine woods treefrog X X Hyla femoralis Barking treefrog X Hyla gratiosa Squirrel treefrog X Hyla squirella Grey treefrog X Hyla chrysoscelis Greenhouse frog X Hyla cinerea Green treefrog X Family Microhylidae—Narrowmouthed Toads Gastrophryne carolinensis Eastern narrowmouth toad X X Family Pelobatidae—Spadefoot Toads Scaphiopus holbrookii holbrookii Eastern spadefoot toad X Family Ranidae—True Frog Family Rana catesbeiana Bullfrog X Rana clamitans clamitans Bronze frog X X Rana sphenocephala Southern leopard frog X X Rana capito gopher frog* X Rana grylio Pig frog X Rana heckscheri River frog X Camp Dresser & McKee *Threatened, endangered, or species of special concern s:\vandyke\lns\munson\t43 4-6 Section 4 Threatened and Endangered Flora and Fauna and Other Wildlife Table 4-3 Common and Scientific Names of Amphibians -

Chirp, Croak, and Snore
MINNESOTA CONSERVATION VOLUNTEER Young Naturalists Teachers Guide Prepared by “Chirp, Croak, and Snore” Multidisciplinary Jack Judkins, Classroom Activities Curriculum Teachers guide for the Young Naturalists article “Chirp, Croak, and Snore” by Mary Hoff. Connections Published in the March–April 2014 Minnesota Conservation Volunteer, or visit www.dnr.state.mn.us/young_naturalists/frogs-and-toads-of-minnesota/index.html. Minnesota Young Naturalists teachers guides are provided free of charge to classroom teachers, parents, and students. This guide contains a brief summary of the article, suggested independent reading levels, word count, materials list, estimates of preparation and instructional time, academic standards applications, preview strategies and study questions overview, adaptations for special needs students, assessment options, extension activities, Web resources (including related Minnesota Conservation Volunteer articles), copy-ready study questions with answer key, and a copy-ready vocabulary sheet and vocabulary study cards. There is also a practice quiz (with answer key) in Minnesota Comprehensive Assessments format. Materials may be reproduced and/or modified to suit user needs. Users are encouraged to provide feedback through an online survey at www.mndnr.gov/education/teachers/activities/ynstudyguides/survey.html. *All Minnesota Conservation Volunteer articles published since 1940 are now online in searchable PDF format. Visit www.mndnr.gov/magazine and click on past issues. Summary “Chirp, Croak, and Snore” surveys -

LCR MSCP Species Accounts, 2008
Lower Colorado River Multi-Species Conservation Program Steering Committee Members Federal Participant Group California Participant Group Bureau of Reclamation California Department of Fish and Game U.S. Fish and Wildlife Service City of Needles National Park Service Coachella Valley Water District Bureau of Land Management Colorado River Board of California Bureau of Indian Affairs Bard Water District Western Area Power Administration Imperial Irrigation District Los Angeles Department of Water and Power Palo Verde Irrigation District Arizona Participant Group San Diego County Water Authority Southern California Edison Company Arizona Department of Water Resources Southern California Public Power Authority Arizona Electric Power Cooperative, Inc. The Metropolitan Water District of Southern Arizona Game and Fish Department California Arizona Power Authority Central Arizona Water Conservation District Cibola Valley Irrigation and Drainage District Nevada Participant Group City of Bullhead City City of Lake Havasu City Colorado River Commission of Nevada City of Mesa Nevada Department of Wildlife City of Somerton Southern Nevada Water Authority City of Yuma Colorado River Commission Power Users Electrical District No. 3, Pinal County, Arizona Basic Water Company Golden Shores Water Conservation District Mohave County Water Authority Mohave Valley Irrigation and Drainage District Native American Participant Group Mohave Water Conservation District North Gila Valley Irrigation and Drainage District Hualapai Tribe Town of Fredonia Colorado River Indian Tribes Town of Thatcher The Cocopah Indian Tribe Town of Wickenburg Salt River Project Agricultural Improvement and Power District Unit “B” Irrigation and Drainage District Conservation Participant Group Wellton-Mohawk Irrigation and Drainage District Yuma County Water Users’ Association Ducks Unlimited Yuma Irrigation District Lower Colorado River RC&D Area, Inc. -

Effects of Oil Sands Process-Affected Water and Substrates on Wood Frog (Rana Sylvatica) Eggs and Tadpoles
Effects of Oil Sands Process-Affected Water and Substrates on Wood Frog (Rana sylvatica) Eggs and Tadpoles By Niti Gupta A Thesis Submitted to the College of Graduate Studies and Research in Partial Fulfillment of the Requirements for the Degree of Master of Science in the Toxicology Graduate Program at the University of Saskatchewan Saskatoon, Saskatchewan, Canada © copyright Niti Gupta, May 2009. All rights reserved. PERMISSION TO USE In presenting this thesis in partial fulfillment of the requirements for a Postgraduate degree from the University of Saskatchewan, I agree that the Libraries of this University may make it freely available for inspection. I further agree that permission for copying of this thesis in any manner, in whole or in part, for scholarly purposes may be granted by the professor or professors who supervised my thesis work or, in their absence, by the Head of the Department or the Dean of the College in which my thesis work was done. It is understood that any copying or publication or use of this thesis or parts thereof for financial gain shall not be allowed without my written permission. It is also understood that due recognition shall be given to me and to the University of Saskatchewan in any scholarly use which may be made of any material in my thesis. Requests for permission to copy or to make other use of material in this thesis in whole or part should be addressed to: Chair of the Toxicology Graduate Program Toxicology Centre University of Saskatchewan 44 Campus Drive Saskatoon, Saskatchewan S7N 5B3 i ABSTRACT An essential element of the reclamation strategy proposed by the oil sands mining industry in northern Alberta, Canada, includes the creation of wetlands for the bioremediation of mining waste materials. -

Frogs of Anderson County, TN When It Comes to Loveable Animals, Frogs Receive High Marks from Kids and Adults Alike
Frogs of Anderson County, TN When it comes to loveable animals, frogs receive high marks from kids and adults alike . There are 14 different kinds of frogs in our area, with some species being much more common than others. A few species are rarely found in Anderson County while others - like the Spring Peeper below - can be heard calling throughout the county. We have not heard one species - the Northern Cricket Frog - in Anderson County since the early 1980s. Learning frog calls is an excellent way to improve your listening skills and is important for helping conserve our local frog populations and other biodiversity. Be sure to check out the Resources Page on the back which lists websites that showcase the calls of Tennessee frogs. Spring Peeper Habitat availability and condition are the two most important factors for determining where frog populations are found — froggy cannot go a-courtin’ without the proper environment! This guide will help you identify our local species and also provide resources for improving or creating good frog habitat. Frogs in our area are classified into one of five families based on their characteristics. For example, 5 of our 14 species belong to the treefrog family which is typically characterized by expanded toe tips for climbing and the ability to vary their coloration. The frogs in this guide are organized by family, but keep in mind that frogs are wonderfully diverse and some species may or may not have physical traits or behaviors which precisely conform to their family characteristics. This schoolyard wetland in Norris, TN is a great habitat for both frogs and kids. -

Herps of the Rio Bosque
The Rio Grande Leopard Frog What is a “herp”? (Rana berlandieri) Herpetology is the branch of zoology has not been seen in the El Paso area for dealing with reptiles and amphibians. many years. Habitat From this formal word, the shorthand term created at the Rio “herps” has come into common use for Bosque would be key to the re-introduction of this referring to these two classes of animals species. together. Both have their origin in the Greek root herpeton: a crawling thing. On the Cover: A Spadefoot Toad sits near a stream. Reptiles and amphibians are all vertebrate tetrapods (“four-legs” – even snakes which “lost” their legs millions of years ago). Wetlands and riverside forests once graced Bullfrog Generally, amphibians produce naked, the banks of the Rio Grande in the Paso del unprotected eggs that must be laid in water Norte region. They were the area’s most to avoid desiccation. Reptile eggs are productive natural habitats, but today they surrounded by protective membranes and of the Rio Bosque are virtually gone. At Rio Bosque Wetlands usually an outer shell, so they can be deposited on land with less risk of drying out. Park, the environment is still changing, but in a new way. Here, a diverse partnership is working to bring back meaningful examples of the unique and valuable ecosystems once Amphibians: The Frogs found in our river valley. At present only one true frog species inhabits the The Rio Bosque Educational Rio Bosque. The Bullfrog (Rana catesbeiana) is Brochure Series was made possible a common denizen of the river valley that is more by a USDA Urban Forestry Grant administered by the Texas Forest Service.