Toxicological Profile for Carbon Tetrachloride
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Toxicological Profile for Carbon Tetrachloride
CARBON TETRACHLORIDE 1 1. PUBLIC HEALTH STATEMENT This public health statement tells you about carbon tetrachloride and the effects of exposure to it. The Environmental Protection Agency (EPA) identifies the most serious hazardous waste sites in the nation. These sites are then placed on the National Priorities List (NPL) and are targeted for long-term federal clean-up activities. Carbon tetrachloride has been found in at least 430 of the 1,662 current or former NPL sites. Although the total number of NPL sites evaluated for this substance is not known, the possibility exists that the number of sites at which carbon tetrachloride is found may increase in the future as more sites are evaluated. This information is important because these sites may be sources of exposure and exposure to this substance may harm you. When a substance is released either from a large area, such as an industrial plant, or from a container, such as a drum or bottle, it enters the environment. Such a release does not always lead to exposure. You can be exposed to a substance only when you come in contact with it. You may be exposed by breathing, eating, or drinking the substance, or by skin contact. If you are exposed to carbon tetrachloride, many factors will determine whether you will be harmed. These factors include the dose (how much), the duration (how long), and how you come in contact with it. You must also consider any other chemicals you are exposed to and your age, sex, diet, family traits, lifestyle, and state of health. -

The Safety Evaluation of Food Flavouring Substances
Toxicology Research View Article Online REVIEW View Journal The safety evaluation of food flavouring substances: the role of metabolic studies Cite this: DOI: 10.1039/c7tx00254h Robert L. Smith,a Samuel M. Cohen, b Shoji Fukushima,c Nigel J. Gooderham,d Stephen S. Hecht,e F. Peter Guengerich, f Ivonne M. C. M. Rietjens,g Maria Bastaki,h Christie L. Harman,h Margaret M. McGowenh and Sean V. Taylor *h The safety assessment of a flavour substance examines several factors, including metabolic and physio- logical disposition data. The present article provides an overview of the metabolism and disposition of flavour substances by identifying general applicable principles of metabolism to illustrate how information on metabolic fate is taken into account in their safety evaluation. The metabolism of the majority of flavour substances involves a series both of enzymatic and non-enzymatic biotransformation that often results in products that are more hydrophilic and more readily excretable than their precursors. Flavours can undergo metabolic reactions, such as oxidation, reduction, or hydrolysis that alter a functional group relative to the parent compound. The altered functional group may serve as a reaction site for a sub- sequent metabolic transformation. Metabolic intermediates undergo conjugation with an endogenous agent such as glucuronic acid, sulphate, glutathione, amino acids, or acetate. Such conjugates are typi- Received 25th September 2017, cally readily excreted through the kidneys and liver. This paper summarizes the types of metabolic reac- Accepted 21st March 2018 tions that have been documented for flavour substances that are added to the human food chain, the DOI: 10.1039/c7tx00254h methodologies available for metabolic studies, and the factors that affect the metabolic fate of a flavour rsc.li/toxicology-research substance. -

The National Drugs List
^ ^ ^ ^ ^[ ^ The National Drugs List Of Syrian Arab Republic Sexth Edition 2006 ! " # "$ % &'() " # * +$, -. / & 0 /+12 3 4" 5 "$ . "$ 67"5,) 0 " /! !2 4? @ % 88 9 3: " # "$ ;+<=2 – G# H H2 I) – 6( – 65 : A B C "5 : , D )* . J!* HK"3 H"$ T ) 4 B K<) +$ LMA N O 3 4P<B &Q / RS ) H< C4VH /430 / 1988 V W* < C A GQ ") 4V / 1000 / C4VH /820 / 2001 V XX K<# C ,V /500 / 1992 V "!X V /946 / 2004 V Z < C V /914 / 2003 V ) < ] +$, [2 / ,) @# @ S%Q2 J"= [ &<\ @ +$ LMA 1 O \ . S X '( ^ & M_ `AB @ &' 3 4" + @ V= 4 )\ " : N " # "$ 6 ) G" 3Q + a C G /<"B d3: C K7 e , fM 4 Q b"$ " < $\ c"7: 5) G . HHH3Q J # Hg ' V"h 6< G* H5 !" # $%" & $' ,* ( )* + 2 ا اوا ادو +% 5 j 2 i1 6 B J' 6<X " 6"[ i2 "$ "< * i3 10 6 i4 11 6! ^ i5 13 6<X "!# * i6 15 7 G!, 6 - k 24"$d dl ?K V *4V h 63[46 ' i8 19 Adl 20 "( 2 i9 20 G Q) 6 i10 20 a 6 m[, 6 i11 21 ?K V $n i12 21 "% * i13 23 b+ 6 i14 23 oe C * i15 24 !, 2 6\ i16 25 C V pq * i17 26 ( S 6) 1, ++ &"r i19 3 +% 27 G 6 ""% i19 28 ^ Ks 2 i20 31 % Ks 2 i21 32 s * i22 35 " " * i23 37 "$ * i24 38 6" i25 39 V t h Gu* v!* 2 i26 39 ( 2 i27 40 B w< Ks 2 i28 40 d C &"r i29 42 "' 6 i30 42 " * i31 42 ":< * i32 5 ./ 0" -33 4 : ANAESTHETICS $ 1 2 -1 :GENERAL ANAESTHETICS AND OXYGEN 4 $1 2 2- ATRACURIUM BESYLATE DROPERIDOL ETHER FENTANYL HALOTHANE ISOFLURANE KETAMINE HCL NITROUS OXIDE OXYGEN PROPOFOL REMIFENTANIL SEVOFLURANE SUFENTANIL THIOPENTAL :LOCAL ANAESTHETICS !67$1 2 -5 AMYLEINE HCL=AMYLOCAINE ARTICAINE BENZOCAINE BUPIVACAINE CINCHOCAINE LIDOCAINE MEPIVACAINE OXETHAZAINE PRAMOXINE PRILOCAINE PREOPERATIVE MEDICATION & SEDATION FOR 9*: ;< " 2 -8 : : SHORT -TERM PROCEDURES ATROPINE DIAZEPAM INJ. -

Specifications of Approved Drug Compound Library
Annexure-I : Specifications of Approved drug compound library The compounds should be structurally diverse, medicinally active, and cell permeable Compounds should have rich documentation with structure, Target, Activity and IC50 should be known Compounds which are supplied should have been validated by NMR and HPLC to ensure high purity Each compound should be supplied as 10mM solution in DMSO and at least 100µl of each compound should be supplied. Compounds should be supplied in screw capped vial arranged as 96 well plate format. -
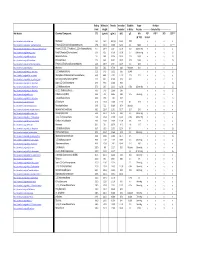
Detectable Compounds by MIP.Pdf
Boiling Molecular Density Ionization Solubility Vapor Analytes Point Weight Potential in Water Pressure ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐Detected By‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Wiki Website Chemical Compound: (oC) (g/mol) (g/mL) (eV) g/L kPa PID* FID** XSD ECD*** @ ~25C 10.6 eV https://en.wikipedia.org/wiki/Methane . Methane ‐161 16.0 .657 g/L 12.61 0.02 n y n n https://en.wikipedia.org/wiki/Dichlorodifluoromethane . Freon 12 (Dichlorodifluoromethane) ‐29.8 120.9 1.486 12.31 0.3 568.0 n y y LS**** https://en.wikipedia.org/wiki/1,1,2‐Trichloro‐1,2,2‐trifluoroethane . Freon 113 (1,1,2‐Trichloro‐1,2,2‐trifluoroethane) 47.7 187.4 1.564 11.78 0.17 285mm Hg n y y y https://en.wikipedia.org/wiki/Vinyl_chloride . Vinyl Chloride (Chloroethene) ‐13.0 62.5 0.910 10.00 2.7 2580mm Hg y y y LS https://en.wikipedia.org/wiki/Bromomethane . Bromomethane 3.6 94.9 3.974 10.53 17.5 190.0 n y y LS https://en.wikipedia.org/wiki/Chloroethane .. Chloroethane 12.3 64.5 0.901 10.97 5.74 134.6 n y y LS https://en.wikipedia.org/wiki/Trichlorofluoromethane . Freon 11 (Trichlorofluoromethane) 23.8 137.4 1.494 11.77 1.1 89.0 n y y y https://en.wikipedia.org/wiki/Acetone . Acetone 56.5 58.1 0.790 9.69 Miscible 30.6 y y n n https://en.wikipedia.org/wiki/1,1‐Dichloroethene . 1,1‐Dichloroethene 32.0 97.0 1.213 9.65 0.04% y y y LS https://en.wikipedia.org/wiki/Dichloromethane . -

COMBINED LIST of Particularly Hazardous Substances
COMBINED LIST of Particularly Hazardous Substances revised 2/4/2021 IARC list 1 are Carcinogenic to humans list compiled by Hector Acuna, UCSB IARC list Group 2A Probably carcinogenic to humans IARC list Group 2B Possibly carcinogenic to humans If any of the chemicals listed below are used in your research then complete a Standard Operating Procedure (SOP) for the product as described in the Chemical Hygiene Plan. Prop 65 known to cause cancer or reproductive toxicity Material(s) not on the list does not preclude one from completing an SOP. Other extremely toxic chemicals KNOWN Carcinogens from National Toxicology Program (NTP) or other high hazards will require the development of an SOP. Red= added in 2020 or status change Reasonably Anticipated NTP EPA Haz list COMBINED LIST of Particularly Hazardous Substances CAS Source from where the material is listed. 6,9-Methano-2,4,3-benzodioxathiepin, 6,7,8,9,10,10- hexachloro-1,5,5a,6,9,9a-hexahydro-, 3-oxide Acutely Toxic Methanimidamide, N,N-dimethyl-N'-[2-methyl-4-[[(methylamino)carbonyl]oxy]phenyl]- Acutely Toxic 1-(2-Chloroethyl)-3-(4-methylcyclohexyl)-1-nitrosourea (Methyl-CCNU) Prop 65 KNOWN Carcinogens NTP 1-(2-Chloroethyl)-3-cyclohexyl-1-nitrosourea (CCNU) IARC list Group 2A Reasonably Anticipated NTP 1-(2-Chloroethyl)-3-cyclohexyl-1-nitrosourea (CCNU) (Lomustine) Prop 65 1-(o-Chlorophenyl)thiourea Acutely Toxic 1,1,1,2-Tetrachloroethane IARC list Group 2B 1,1,2,2-Tetrachloroethane Prop 65 IARC list Group 2B 1,1-Dichloro-2,2-bis(p -chloropheny)ethylene (DDE) Prop 65 1,1-Dichloroethane -

Chemical Hygiene Plan Manual
CHEMICAL HYGIENE PLAN AND HAZARDOUS MATERIALS SAFETY MANUAL FOR LABORATORIES This is the Chemical Hygiene Plan specific to the following areas: Laboratory name or room number(s): ___________________________________ Building: __________________________________________________________ Supervisor: _______________________________________________________ Department: _______________________________________________________ Telephone numbers 911 for Emergency and urgent consultation 48221 Police business line 46919 Fire Dept business line 46371 Radiological and Environmental Management Revisied on: Enter a revision date here. All laboratory chemical use areas must maintain a work-area specific Chemical Hygiene Plan which conforms to the requirements of the OSHA Laboraotry Standard 29 CFR 19190.1450. Purdue University laboratories may use this document as a starting point for creating their work area specific CHP. Minimally this cover page is to be edited for work area specificity (non-West Lafayette laboratories are to place their own emergency, fire, and police telephone numbers in the space above) AND appendix K must be completed. This instruction and information box should remain. This model CHP is version 2010A; updates are to be found at www.purdue.edu/rem This page intentionally blank. PURDUE CHEMICAL HYGIENE PLAN AWARENESS CERTIFICATION For CHP of: ______________________________ Professor, building, rooms The Occupational Safety and Health Administration (OSHA) requires that laboratory employees be made aware of the Chemical Hygiene Plan at their place of employment (29 CFR 1910.1450). The Purdue University Chemical Hygiene Plan and Hazardous Materials Safety Manual serves as the written Chemical Hygiene Plan (CHP) for laboratories using chemicals at Purdue University. The CHP is a regular, continuing effort, not a standby or short term activity. Departments, divisions, sections, or other work units engaged in laboratory work whose hazards are not sufficiently covered in this written manual must customize it by adding their own sections as appropriate (e.g. -

Dichlorodifluoromethane Dcf
DICHLORODIFLUOROMETHANE DCF CAUTIONARY RESPONSE INFORMATION 4. FIRE HAZARDS 7. SHIPPING INFORMATION 4.1 Flash Point: 7.1 Grades of Purity: 99.5% (vol.) Common Synonyms Gas Colorless Faint odor Not flammable 7.2 Storage Temperature: Ambient Arcton 6 4.2 Flammable Limits in Air: Not flammable Eskimon 12 7.3 Inert Atmosphere: No requirement 4.3 Fire Extinguishing Agents: Not F-12 Visible vapor cloud is produced. 7.4 Venting: Safety relief flammable Freon 12 7.5 IMO Pollution Category: Currently not available Frigen 12 4.4 Fire Extinguishing Agents Not to Be Genetron 12 Used: Not flammable 7.6 Ship Type: Currently not available Halon 122 4.5 Special Hazards of Combustion 7.7 Barge Hull Type: 3 Isotron 12 Products: Although nonflammable, Ucon 12 dissociation products generated in a fire may be irritating or toxic. 8. HAZARD CLASSIFICATIONS Notify local health and pollution control agencies. 4.6 Behavior in Fire: Helps extinguish fire. 8.1 49 CFR Category: Nonflammable gas Avoid inhalation. 4.7 Auto Ignition Temperature: Not 8.2 49 CFR Class: 2.2 flammable 8.3 49 CFR Package Group: Not pertinent. Not flammable. 4.8 Electrical Hazards: Not pertinent Fire Cool exposed containers with water. 8.4 Marine Pollutant: No 4.9 Burning Rate: Not flammable 8.5 NFPA Hazard Classification: Not listed 4.10 Adiabatic Flame Temperature: Currently 8.6 EPA Reportable Quantity: 5000 pounds CALL FOR MEDICAL AID. not available Exposure 8.7 EPA Pollution Category: D 4.11 Stoichometric Air to Fuel Ratio: Not VAPOR 8.8 RCRA Waste Number: U075 Not irritating to eyes, nose or throat. -

1. Two Components, Two Sets of Lecturers
Conditions 1. Two components, two sets of lecturers. 2. Lectures 1-5 Prof. F. Hudecz Lectures 6-9 Dr. Gy. Domány Lectures 10-12 Dr. P. Buzder-Lantos 3. Examination: two parts determined by the lecturers and one mark. - option A: written test - option B: presentation based on literature - option C: oral examination 4. Participation at lectures > 70 % [email protected] Some Approved Peptide Pharmaceuticals and their Methods of Manufacture First generatioin Second generation New generation Oxytocin (L) Carbetocin (S) Abarelix (GnRH) (L) ACTH (1-24) & (1-39) (L,S) Terlipressin (L,S) Cetrorelix (GnRH) (L) Vasopressin (L,S) Felypressin (L,S) Ganirelix (GnRH) (L) Insulin (E,SS, R) Buserelin (L,S) Eptifibatide Glucagon (E,S,R) Deslorelin (L,S) Bivalirudin (L) Calcitonins (L,S,R) Goserelin (L) Copaxone (L) TRH (L) Histrelin (L) Techtide P-289(S) Gonadorelin (L,S) Leuprolide (L,S) Cubicin (F) Somatostatin (L,S) Nafarelin (S) Fuzeon (antiHIV (H) GHRH (1-29) & (1-44) (S) Tryptorelin (L,S) Ziconotide (pain) (S) CRF (Human & Ovine) (S) Lecirelin (S) Pramlintide (diabetes) (S) Cyclosporin (F) Lanreotide (S) Exenatide (diabetes) (S) Thymopentin (L) Octreotide (L,S) Icatibant (brady-rec) Thymosin Alpha-1 (S) Atosiban (L) Romiplostim (hormon) Secretins (Human & Porcine) (E,S) Desmopressin (L,S) Degarelix (GnRH) Parathyroid Hormone (1-34) & (1-84)(S) Lypressin (L) Mifamurtide (rák, adj.) Vasoactive Intestinal Polypeptide (S) Ornipressin Ecallantide (ödéma) Brain Natriuretic Peptide (R) Pitressin (L) Liraglutide (diabetes) Cholecystokinin (L) ACE Inhibitors (Enalapril, Lisinopril) (L) Tesamorelin Tetragastrin (L) HIV Protease Inhibitors (L) Surfaxin Pentagastrin (L) Peginesatide Eledoisin (L) Carfilzomib Linaclotide (enz.inh) L = in solution; S = on solid phase; E = extraction; F = fermentation; H = hybrid synthesis; R = recombinant; SS = semi-synthesis. -

Exposure Investigation Protocol: the Identification of Air Contaminants Around the Continental Aluminum Plant in New Hudson, Michigan Conducted by ATSDR and MDCH
Exposure Investigation Protocol - Continental Aluminum New Hudson, Lyon Township, Oakland County, Michigan Exposure Investigation Protocol: The Identification of Air Contaminants Around the Continental Aluminum Plant in New Hudson, Michigan Conducted by ATSDR and MDCH MDCH/ATSDR - 2004 Exposure Investigation Protocol - Continental Aluminum New Hudson, Lyon Township, Oakland County, Michigan TABLE OF CONTENTS OBJECTIVE/PURPOSE..................................................................................................... 3 RATIONALE...................................................................................................................... 4 BACKGROUND ................................................................................................................ 5 AGENCY ROLES .............................................................................................................. 6 ESTABLISHING CRITERIA ............................................................................................ 7 “Odor Events”................................................................................................................. 7 Comparison Values......................................................................................................... 7 METHODS ....................................................................................................................... 12 Instantaneous (“Grab”) Air Sampling........................................................................... 12 Continuous Air Monitoring.......................................................................................... -

(Esi Scheme) Laxminagar, Ajmer Road, Jaipur-302006
DIRECTORATE, MEDICAL & HEALTH SERVICES, (E.S.I. SCHEME) LAXMINAGAR, AJMER ROAD, JAIPUR-302006 Tel/Fax 0141-2223572,www.health.rajasthan.gov.in/esi E-Mail [email protected],[email protected] Ref. No. F434/ESI/Store/LabEmp/2017/5540 Dated:01/11/2017 E- Tender Document For “E- Tender forAnalysis of Drugs, Cotton, Gauzes, Bandage & Other items” For The Office of the Director cum Ex-officio Dy. Secretary, Medical & Health Services ESI Scheme Rajasthan, Laxmi Nagar, Ajmer Road, Jaipur & attached ESIS Institutions in Rajasthan ( for the period of two years from the date of award. ) ( Non-transferable ) Price of Tender Document Rs. 2000/- Last date & time of submission of Technical & Financial Bids is 11.12.17 at 1:30 pm Date & time of online opening of Technical Bid is 11.12.17 at 3:00 pm Visit Us At http://sppp.raj.nic.in, www.dipronline.org, http://eproc.rajasthan.gov.in, www.health.rajasthan.gov.in/esi Our Address: DIRECTORATE, MEDICAL & HEALTH SERVICES, (E.S.I. SCHEME) LAXMINAGAR, AJMER ROAD, JAIPUR-302006 1/71 DIRECTORATE, MEDICAL & HEALTH SERVICES, (E.S.I. SCHEME) LAXMINAGAR, AJMER ROAD, JAIPUR-302006 Tel/Fax 0141-2223572,www.health.rajasthan.gov.in/esi E-Mail [email protected],[email protected] Ref.No.F434/ESI/Store/LabEmp/2017/ 5540 Dated:01/11/2017 E-bids in two separate bids (Technical & Financial) for the Empanelment of Analytic Testing Laboratories For The Test and Analysis of drugs, cotton, bandage, gauze & other items. Brief Details of E-Tender Estimated Cost : Rs 50 lacs EMD (Bid Security)Rs 100000/- RISL processing fees Rs 1000/- Cost of the Tender Document Rs 2000/- Performance Security 5% of contract value Services Required:- The service of analysis of drugs, cotton, gauze, bandages & other items are required in the office of the Director cum Ex- officio Dy. -

Refrigerant Safety Refrigerant History
Refrigerant Safety The risks associated with the use of refrigerants in refrigeration and airconditioning equipment can include toxicity, flammability, asphyxiation, and physical hazards. Although refrigerants can pose one or more of these risks, system design, engineering controls, and other techniques mitigate this risk for the use of refrigerant in various types of equipment. Refrigerant History Nearly all of the historically used refrigerants were flammable, toxic, or both. Some were also highly reactive, resulting in accidents (e. g., leak, explosion) due to equipment failure, poor maintenance, or human error. The task of finding a nonflammable refrigerant with good stability was given to Thomas Midgley in 1926. With his associates Albert Leon Henne and Robert Reed McNary, Dr. Midgley observed that the refrigerants then in use comprised relatively few chemical elements, many of which were clustered in an intersecting row and column of the periodic table of elements. The element at the intersection was fluorine, known to be toxic by itself. Midgley and his collaborators felt, however, that compounds containing fluorine could be both nontoxic and nonflammable. The attention of Midgley and his associates was drawn to organic fluorides by an error in the literature that showed the boiling point for tetrafluoromethane (carbon tetrafluoride) to be high compared to those for other fluorinated compounds. The correct boiling temperature later was found to be much lower. Nevertheless, the incorrect value was in the range sought and led to evaluation of organic fluorides as candidates. The shorthand convention, later introduced to simplify identification of the organic fluorides for a systematic search, is used today as the numbering system for refrigerants.