The Emergence of Compartmental Organization in the Olfactory Bulb Glomerulus
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ultrastructural Study of the Granule Cell Domain of the Cochlear Nucleus in Rats: Mossy Fiber Endings and Their Targets
THE JOURNAL OF COMPARATIVE NEUROLOGY 369~345-360 ( 1996) Ultrastructural Study of the Granule Cell Domain of the Cochlear Nucleus in Rats: Mossy Fiber Endings and Their Targets DIANA L. WEEDMAN, TAN PONGSTAPORN, AND DAVID K. RYUGO Center for Hearing Sciences, Departments of Otolaryngoloby-Head and Neck Surgery and Neuroscience, Johns Hopkins University School of Medicine, Baltimore, Maryland 2 1205 ABSTRACT The principal projection neurons of the cochlear nucleus receive the bulk of their input from the auditory nerve. These projection neurons reside in the core of the nucleus and are surrounded by an external shell, which is called the granule cell domain. Interneurons of the cochlear granule cell domain are the target for nonprimary auditory inputs, including projections from the superior olivary complex, inferior colliculus, and auditory cortex. The granule cell domain also receives projections from the cuneate and trigeminal nuclei, which are first-order nuclei of the somatosensory system. The cellular targets of the nonprimary projections are mostly unknown due to a lack of information regarding postsynaptic profiles in the granule cell areas. In the present paper, we examined the synaptic relationships between a heterogeneous class of large synaptic terminals called mossy fibers and their targets within subdivisions of the granule cell domain known as the lamina and superficial layer. By using light and electron microscopic methods in these subdivisions, we provide evidence for three different neuron classes that receive input from the mossy fibers: granule cells, unipolar brush cells, and a previously undescribed class called chestnut cells. The distinct synaptic relations between mossy fibers and members of each neuron class further imply fundamentally separate roles for processing acoustic signals. -

Biophysical Network Modelling of the Dlgn Circuit: Different Effects of Triadic and Axonal Inhibition on Visual Responses of Relay Cells
RESEARCH ARTICLE Biophysical Network Modelling of the dLGN Circuit: Different Effects of Triadic and Axonal Inhibition on Visual Responses of Relay Cells Thomas Heiberg1, Espen Hagen1,2, Geir Halnes1, Gaute T. Einevoll1,3* 1 Department of Mathematical Sciences and Technology, Norwegian University of Life Sciences, Ås, Norway, 2 Institute of Neuroscience and Medicine (INM-6) and Institute for Advanced Simulation (IAS-6) and a11111 JARA BRAIN Institute I, Jülich Research Centre, Jülich, Germany, 3 Department of Physics, University of Oslo, Oslo, Norway * [email protected] Abstract OPEN ACCESS Despite its prominent placement between the retina and primary visual cortex in the early Citation: Heiberg T, Hagen E, Halnes G, Einevoll GT visual pathway, the role of the dorsal lateral geniculate nucleus (dLGN) in molding and regu- (2016) Biophysical Network Modelling of the dLGN lating the visual signals entering the brain is still poorly understood. A striking feature of the Circuit: Different Effects of Triadic and Axonal Inhibition on Visual Responses of Relay Cells. PLoS dLGN circuit is that relay cells (RCs) and interneurons (INs) form so-called triadic synapses, Comput Biol 12(5): e1004929. doi:10.1371/journal. where an IN dendritic terminal can be simultaneously postsynaptic to a retinal ganglion cell pcbi.1004929 (GC) input and presynaptic to an RC dendrite, allowing for so-called triadic inhibition. Taking Editor: Arnd Roth, University College London, advantage of a recently developed biophysically detailed multicompartmental model for an UNITED KINGDOM IN, we here investigate putative effects of these different inhibitory actions of INs, i.e., triadic Received: August 29, 2015 inhibition and standard axonal inhibition, on the response properties of RCs. -

1 Evidence for Gliadin Antibodies As Causative Agents in Schizophrenia
1 Evidence for gliadin antibodies as causative agents in schizophrenia. C.J.Carter PolygenicPathways, 20 Upper Maze Hill, Saint-Leonard’s on Sea, East Sussex, TN37 0LG [email protected] Tel: 0044 (0)1424 422201 I have no fax Abstract Antibodies to gliadin, a component of gluten, have frequently been reported in schizophrenia patients, and in some cases remission has been noted following the instigation of a gluten free diet. Gliadin is a highly immunogenic protein, and B cell epitopes along its entire immunogenic length are homologous to the products of numerous proteins relevant to schizophrenia (p = 0.012 to 3e-25). These include members of the DISC1 interactome, of glutamate, dopamine and neuregulin signalling networks, and of pathways involved in plasticity, dendritic growth or myelination. Antibodies to gliadin are likely to cross react with these key proteins, as has already been observed with synapsin 1 and calreticulin. Gliadin may thus be a causative agent in schizophrenia, under certain genetic and immunological conditions, producing its effects via antibody mediated knockdown of multiple proteins relevant to the disease process. Because of such homology, an autoimmune response may be sustained by the human antigens that resemble gliadin itself, a scenario supported by many reports of immune activation both in the brain and in lymphocytes in schizophrenia. Gluten free diets and removal of such antibodies may be of therapeutic benefit in certain cases of schizophrenia. 2 Introduction A number of studies from China, Norway, and the USA have reported the presence of gliadin antibodies in schizophrenia 1-5. Gliadin is a component of gluten, intolerance to which is implicated in coeliac disease 6. -
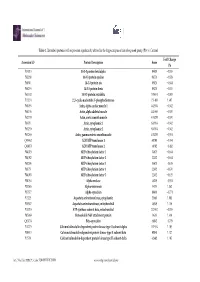
Table 1. Identified Proteins with Expression Significantly Altered in the Hippocampus of Rats of Exposed Group (Pb) Vs
Table 1. Identified proteins with expression significantly altered in the hippocampus of rats of exposed group (Pb) vs. Control. Fold Change Accession Id a Protein Description Score Pb P35213 14-3-3 protein beta/alpha 85420 −0.835 P62260 14-3-3 protein epsilon 96570 −0.878 P68511 14-3-3 protein eta 85420 −0.844 P68255 14-3-3 protein theta 85420 −0.835 P63102 14-3-3 protein zeta/delta 105051 −0.803 P13233 2',3'-cyclic-nucleotide 3'-phosphodiesterase 151400 1.405 P68035 Actin, alpha cardiac muscle 1 442584 −0.942 P68136 Actin, alpha skeletal muscle 441060 −0.970 P62738 Actin, aortic smooth muscle 438270 −0.970 P60711 Actin, cytoplasmic 1 630104 −0.942 P63259 Actin, cytoplasmic 2 630104 −0.942 P63269 Actin, gamma-enteric smooth muscle 438270 −0.951 Q05962 ADP/ATP translocase 1 60100 −0.554 Q09073 ADP/ATP translocase 2 49102 −0.482 P84079 ADP-ribosylation factor 1 34675 −0.644 P84082 ADP-ribosylation factor 2 22412 −0.644 P61206 ADP-ribosylation factor 3 34675 −0.619 P61751 ADP-ribosylation factor 4 22412 −0.670 P84083 ADP-ribosylation factor 5 22412 −0.625 P04764 Alpha-enolase 46219 −0.951 P23565 Alpha-internexin 9478 1.062 P37377 Alpha-synuclein 89619 −0.771 P13221 Aspartate aminotransferase, cytoplasmic 23661 1.083 P00507 Aspartate aminotransferase, mitochondrial 46049 1.116 P10719 ATP synthase subunit beta, mitochondrial 232442 −0.835 P85969 Beta-soluble NSF attachment protein 9638 1.419 Q63754 Beta-synuclein 66842 −0.779 P11275 Calcium/calmodulin-dependent protein kinase type II subunit alpha 181954 1.105 P08413 Calcium/calmodulin-dependent protein kinase type II subunit beta 80840 1.127 P15791 Calcium/calmodulin-dependent protein kinase type II subunit delta 62682 1.105 Int. -

1 Metabolic Dysfunction Is Restricted to the Sciatic Nerve in Experimental
Page 1 of 255 Diabetes Metabolic dysfunction is restricted to the sciatic nerve in experimental diabetic neuropathy Oliver J. Freeman1,2, Richard D. Unwin2,3, Andrew W. Dowsey2,3, Paul Begley2,3, Sumia Ali1, Katherine A. Hollywood2,3, Nitin Rustogi2,3, Rasmus S. Petersen1, Warwick B. Dunn2,3†, Garth J.S. Cooper2,3,4,5* & Natalie J. Gardiner1* 1 Faculty of Life Sciences, University of Manchester, UK 2 Centre for Advanced Discovery and Experimental Therapeutics (CADET), Central Manchester University Hospitals NHS Foundation Trust, Manchester Academic Health Sciences Centre, Manchester, UK 3 Centre for Endocrinology and Diabetes, Institute of Human Development, Faculty of Medical and Human Sciences, University of Manchester, UK 4 School of Biological Sciences, University of Auckland, New Zealand 5 Department of Pharmacology, Medical Sciences Division, University of Oxford, UK † Present address: School of Biosciences, University of Birmingham, UK *Joint corresponding authors: Natalie J. Gardiner and Garth J.S. Cooper Email: [email protected]; [email protected] Address: University of Manchester, AV Hill Building, Oxford Road, Manchester, M13 9PT, United Kingdom Telephone: +44 161 275 5768; +44 161 701 0240 Word count: 4,490 Number of tables: 1, Number of figures: 6 Running title: Metabolic dysfunction in diabetic neuropathy 1 Diabetes Publish Ahead of Print, published online October 15, 2015 Diabetes Page 2 of 255 Abstract High glucose levels in the peripheral nervous system (PNS) have been implicated in the pathogenesis of diabetic neuropathy (DN). However our understanding of the molecular mechanisms which cause the marked distal pathology is incomplete. Here we performed a comprehensive, system-wide analysis of the PNS of a rodent model of DN. -

Granule Cell Synapses of Rat Cerebellum: Experimental Observations and Theoretical Predictions
Articles in PresS. J Neurophysiol (October 5, 2005). doi:10.1152/jn.00696.2005 1 LTP regulates burst initiation and frequency at mossy fiber – granule cell synapses of rat cerebellum: experimental observations and theoretical predictions Thierry Nieus1,2, Elisabetta Sola1,4, Jonathan Mapelli1, Elena Saftenku3, Paola Rossi1, Egidio D’Angelo1,2,* 1 Dept. of Cellular-Molecular Physiological and Pharmacological Sciences (University of Pavia) and INFM, Via Forlanini 6, I-27100, Pavia, Italy. 2 Dept. of Functional and Evolutionary Biology (University of Parma), Parco Area delle Scienze 11a, I-34100, Parma, Italy. 3 Dept. of General Physiology of Nervous System, A.A. Bogomoletz Institute of Physiology Bogomoletz St., 4, 01024, Kiev-24, Ukraine 4 present address: Dept. of Biophysics, SISSA, via Beirut 4, I-34014, Trieste, Italy * To whom correspondence should be addressed at [email protected] Key-words. LTP, Synaptic plasticity, Presynaptic mechanisms, Cerebellum, Granule cells Running title. LTP and bursting in the cerebellum Acknowledgments. This work was supported by projects of the European Community (CEREBELLUM QLG3-CT-2001-02256 and SPIKEFORCE IST-2001-35271), of MIUR and INFM of Italy to ED. ABSTRACT Long-term potentiation (LTP) is a synaptic change supposed to provide the cellular basis for learning and memory in brain neuronal circuits. Although specific LTP expression mechanisms could be critical to determine the dynamics of repetitive neurotransmission, this important issue remained largely unexplored. In this paper we have performed whole-cell patch-clamp recordings of mossy fiber – granule cell LTP in acute rat cerebellar slices and investigated its computational implications with a mathematical model. During LTP, stimulation with short impulse trains at 100 Hz revealed earlier initiation of granule cell spike bursts and a smaller non-significant spike frequency increase. -

Identification of a Mutation in Synapsin I, a Synaptic Vesicle Protein, in a Family with Epilepsy J Med Genet: First Published As on 1 March 2004
183 SHORT REPORT Identification of a mutation in synapsin I, a synaptic vesicle protein, in a family with epilepsy J Med Genet: first published as on 1 March 2004. Downloaded from C C Garcia, H J Blair, M Seager, A Coulthard, S Tennant, M Buddles, A Curtis, J A Goodship ............................................................................................................................... J Med Genet 2004;41:183–187. doi: 10.1136/jmg.2003.013680 hippocampal bodies are moderately atrophic but without A four generation family is described in which some men of signal intensities. normal intelligence have epilepsy and others have various III-2 is a 64 year old man who has had a cerebrovascular combinations of epilepsy, learning difficulties, macrocephaly, accident but was previously of normal intelligence. He had and aggressive behaviour. As the phenotype in this family is tonic-clonic seizures until aged 7. His head circumference is distinct from other X linked recessive disorders linkage studies normal. were carried out. Linkage analysis was done using X III-3 is a 53 year old man with a full scale IQ that has been chromosome microsatellite polymorphisms to define the formally assessed as 72 (with the recognised error in such interval containing the causative gene. Genes from within assessments this places him within the borderline to mild the region were considered possible candidates and one of learning difficulties range). His head circumference is these, SYN1, was screened for mutations by direct DNA normal. As a teenager, he showed extreme physical aggres- sequencing of amplified products. Microsatellite analysis sion (he was held in a high security psychiatric hospital showed that the region between MAOB (Xp11.3) and between the ages of 11 and 18). -

The Olfactory Receptor Associated Proteome
INTERNATIONAL GRADUATE SCHOOL OF NEUROSCIENCES (IGSN) RUHR UNIVERSITÄT BOCHUM THE OLFACTORY RECEPTOR ASSOCIATED PROTEOME Doctoral Dissertation David Jonathan Barbour Department of Cell Physiology Thesis advisor: Prof. Dr. Dr. Dr. Hanns Hatt Bochum, Germany (30.12.05) ABSTRACT Olfactory receptors (OR) are G-protein-coupled membrane receptors (GPCRs) that comprise the largest vertebrate multigene family (~1,000 ORs in mouse and rat, ~350 in human); they are expressed individually in the sensory neurons of the nose and have also been identified in human testis and sperm. In order to gain further insight into the underlying molecular mechanisms of OR regulation, a bifurcate proteomic strategy was employed. Firstly, the question of stimulus induced plasticity of the olfactory sensory neuron was addressed. Juvenile mice were exposed to either a pulsed or continuous application of an aldehyde odorant, octanal, for 20 days. This was followed by behavioural, electrophysiological and proteomic investigations. Both treated groups displayed peripheral desensitization to octanal as determined by electro-olfactogram recordings. This was not due to anosmia as they were on average faster than the control group in a behavioural food discovery task. To elucidate differentially regulated proteins between the control and treated mice, fluorescent Difference Gel Electrophoresis (DIGE) was used. Seven significantly up-regulated and ten significantly down-regulated gel spots were identified in the continuously treated mice; four and twenty-four significantly up- and down-regulated spots were identified for the pulsed mice, respectively. The spots were excised and proteins were identified using mass spectrometry. Several promising candidate proteins were identified including potential transcription factors, cytoskeletal proteins as well as calcium binding and odorant binding proteins. -
Synuclein Reduces Neurotransmitter Release by Inhibiting Synaptic Vesicle Reclustering After Endocytosis
Neuron Article Increased Expression of a-Synuclein Reduces Neurotransmitter Release by Inhibiting Synaptic Vesicle Reclustering after Endocytosis Venu M. Nemani,1 Wei Lu,2 Victoria Berge,3 Ken Nakamura,1 Bibiana Onoa,1 Michael K. Lee,4 Farrukh A. Chaudhry,3 Roger A. Nicoll,2 and Robert H. Edwards1,* 1Departments of Neurology and Physiology, Graduate Program in Neuroscience 2Department of Cellular and Molecular Pharmacology University of California, San Francisco, San Francisco, CA 94158, USA 3The Biotechnology Centre of Oslo, Centre for Molecular Biology and Neuroscience, University of Oslo, Oslo, Norway 4Department of Pathology, Johns Hopkins University School of Medicine, Baltimore, MD 21205, USA *Correspondence: [email protected] DOI 10.1016/j.neuron.2009.12.023 SUMMARY Parkinson’s disease (PD) and Lewy body dementia (LBD) (Spill- antini et al., 1998; Dickson, 2001). Point mutations in asyn are The protein a-synuclein accumulates in the brain of also linked to an autosomal-dominant form of PD (Polymeropou- patients with sporadic Parkinson’s disease (PD), los et al., 1997; Kru¨ ger et al., 1998; Zarranz et al., 2004). Taken and increased gene dosage causes a severe, domi- together, these observations suggest a causative role for asyn nantly inherited form of PD, but we know little about in sporadic as well as inherited PD and LBD. In addition, duplica- the effects of synuclein that precede degeneration. tion and triplication of the asyn gene suffice to cause a severe, a-Synuclein localizes to the nerve terminal, but the highly penetrant form of PD (Singleton et al., 2003; Chartier- Harlin et al., 2004), and polymorphisms in regulatory elements knockout has little if any effect on synaptic transmis- of the asyn gene predispose to PD (Maraganore et al., 2006), sion. -

Three-Dimensional Morphology of Cerebellar Protoplasmic Islands
Scanning Microscopy Volume 5 Number 2 Article 16 2-16-1991 Three-Dimensional Morphology of Cerebellar Protoplasmic Islands and Proteoglycan Content of Mossy Fiber Glomerulus: A Scanning and Transmission Electron Microscope Study O. J. Castejón Universidad del Zulia, Venezuela H. V. Castejón Universidad del Zulia, Venezuela Follow this and additional works at: https://digitalcommons.usu.edu/microscopy Part of the Biology Commons Recommended Citation Castejón, O. J. and Castejón, H. V. (1991) "Three-Dimensional Morphology of Cerebellar Protoplasmic Islands and Proteoglycan Content of Mossy Fiber Glomerulus: A Scanning and Transmission Electron Microscope Study," Scanning Microscopy: Vol. 5 : No. 2 , Article 16. Available at: https://digitalcommons.usu.edu/microscopy/vol5/iss2/16 This Article is brought to you for free and open access by the Western Dairy Center at DigitalCommons@USU. It has been accepted for inclusion in Scanning Microscopy by an authorized administrator of DigitalCommons@USU. For more information, please contact [email protected]. Scanning Microscopy , Vol. 5, No . 2, 1991 (Pages 477-494) 0891 -7035/91$3.00+ .00 Scanning Microscopy International , Chicago (AMF O'Hare) , IL 60666 USA THREE-DI MENSIONAL MORPHOLOGYOF CEREBELLAR PROTOPLASMIC ISLANDS AND PROTEOGLYCANCO NTENT OF MOSSY FIBER GLOMERULUS: A SCANNING AND TRANSMISSION ELECTRON MICROSCOPE STUDY 0. J . Cas tejon and H. V. Castejon Institute de Investigac1ones Biologicas. Facultad de Medicina . Universidad de l Zulia. Apart ado 526, Mara ca ibo, Venezu ela (Received for publication May 2, 1990 , and in revised form February 16, 1991) Abstract Introduction The present rev , ew summarizes the outer and The cerebellar protoplasmatic islands and inner surface features of mossy f ib er glomeru li the cerebellar glomeruli were studied at light 1n vertebra te cerebellar granular la yer as seen microscope level by Denissenko ( 18771, Ramon y by conv en t i ona l scanning electron microscopy Cajal ( 1889, 19551, Retzius ( 1892a,b), Lugaro <SEMI and SEM fre eze -f ra cture method . -

Synaptic Ultrastructure of Functionally and Morphologically Characterized Neurons of the Superficial Spinal Dorsal Horn Cat
The Journal of Neuroscience, June 1989, g(8): 1848-l 883 Synaptic Ultrastructure of Functionally and Morphologically Characterized Neurons of the Superficial Spinal Dorsal Horn Cat M. Wthelyi, A. R. Light, and E. Ft. Per1 Department of Physiology, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina 27599, and Second Department of Anatomy, Semmelweis University, Budapest, Hungary Recordings of neuronal unitary discharges evoked by pri- neurons, regardless of gross configuration, were found to mary afferent input were made in the superficial part of the have vesicles in their dendrites, but 3 nocireceptiveneurons spinal cord’s dorsal horn, the marginal zone and substantia received synapses from presynaptic dendritic profiles. gelatinosa (also known as laminae I and II) , using fine mi- cropipette electrodes filled with HRP. After physiological The superficial dorsal horn, laminae I and II, of the spinal cord characterization with respect to primary afferent input, HRP (Brown and Rethelyi, 1981) receivesprimary afferent input prin- was injected intracellularly iontophoretically into the record- cipally or exclusively from fine fibers, myelinated and unmy- ed neuron. Following histochemical processing, the neurons elinated, that pass through the lateral division of the dorsal so delineated were studied at the light and electron micro- rootlets (Sindou et al., 1974; Snyder, 1977; Light and Perl, 1979a, scopic levels. No clear relationship between function and b). However, from the functional standpoint, the input to the either general cellular configuration or synaptic ultrastruc- region from the periphery is diverse (Kumazawa and Perl, 1978; ture appeared in these analyses, although the concentration Rethelyi et al., 1979; Perl, 1984). There have been numerous of dendritic distribution could be related to the nature of descriptions of the structure of neurons and terminal axon sys- primary afferent excitation. -

Astroglial Glutamate Transporters in the Brain: Regulating Neurotransmitter Homeostasis and Synaptic Transmission
Journal of Neuroscience Research 95:2140–2151 (2017) Review Astroglial Glutamate Transporters in the Brain: Regulating Neurotransmitter Homeostasis and Synaptic Transmission Ciaran Murphy-Royal,1,2 Julien Dupuis,2,3 Laurent Groc,2,3 and Stephane H. R. Oliet 1,2 1Neurocentre Magendie, Inserm U1215, Bordeaux, France 2Universite de Bordeaux, Bordeaux, France 3Interdisciplinary Institute for Neuroscience, CNRS UMR 5297, Bordeaux, France Astrocytes, the major glial cell type in the central nervous compartments. As an example, the extracellular glutamate system (CNS), are critical for brain function and have concentration is maintained at low values lying most like- been implicated in various disorders of the central ner- ly below 100 nM (Hamberger and Nystrom,€ 1984; vous system. These cells are involved in a wide range of Cavelier and Attwell, 2005; Herman and Jahr, 2007), cerebral processes including brain metabolism, control of while it is much more concentrated inside cells. As there central blood flow, ionic homeostasis, fine-tuning synap- are no extracellular enzymes degrading glutamate, this tic transmission, and neurotransmitter clearance. Such low extracellular concentration can be fully accounted for varied roles can be efficiently carried out due to the inti- by specific transporters, which are widely expressed mate interactions astrocytes maintain with neurons, the throughout the brain (Furuta et al., 1997). Glutamate vasculature, as well as with other glial cells. Arguably, one transporters working under physiological conditions have of the most important functions of astrocytes in the brain the capacity to establish concentration gradients of 106 is their control of neurotransmitter clearance. This is across the plasma membrane, theoretically resulting in a particularly true for glutamate whose timecourse in the glutamate concentration of 10 nM extracellularly and 10 synaptic cleft needs to be controlled tightly under physio- mM intracellularly (Zerangue and Kavanaugh, 1996a).