The EC-HDA9 Complex Rhythmically Regulates Histone Acetylation at the TOC1 Promoter in Arabidopsis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Molecular Elucidation of Potato Tuberisation Pathway
MOLECULAR ELUCIDATION OF POTATO TUBERISATION PATHWAY Kirika, Margaret Plant Breeding Department Wageningen UR 8/11/2014 Table of Contents 1.0 Introduction ........................................................................................................................................ 3 1.1 CONSTANS/FLOWERING LOCUS T (CO/FT) MODULE ............................................................. 5 1.2 Molecular pathways regulating tuberisation in potato .................................................................... 7 1.3 Potato FT homologs StSP5G and StSP6A involvement in tuberisation ........................................ 8 1.4 Gibberellin role in tuberisation ....................................................................................................... 8 1.5 StPOTH1, StBEL5 and miR172 ..................................................................................................... 9 1.6 Potato StCO1/2 ............................................................................................................................ 11 1.6.1 Daily oscillation of StCO ........................................................................................................... 12 1.7 StCDF1 a central regulator of tuberisation .................................................................................. 12 1.7.1 Localization of CDF1 ................................................................................................................. 13 1.8 Rationale ..................................................................................................................................... -

The Plant Circadian Clock and Chromatin Modifications
G C A T T A C G G C A T genes Review The Plant Circadian Clock and Chromatin Modifications Ping Yang 1,2, Jianhao Wang 1,2, Fu-Yu Huang 3 , Songguang Yang 1,* and Keqiang Wu 3,* 1 Key Laboratory of South China Agricultural Plant Molecular Analysis and Genetic Improvement, South China Botanical Garden, Chinese Academy of Sciences, Guangzhou 510650, China; [email protected] (P.Y.); [email protected] (J.W.) 2 University of Chinese Academy of Sciences, Chinese Academy of Sciences, Beijing 100049, China 3 Institute of Plant Biology, National Taiwan University, Taipei 106, Taiwan; [email protected] * Correspondence: [email protected] (S.Y.); [email protected] (K.W.) Received: 3 October 2018; Accepted: 5 November 2018; Published: 20 November 2018 Abstract: The circadian clock is an endogenous timekeeping network that integrates environmental signals with internal cues to coordinate diverse physiological processes. The circadian function depends on the precise regulation of rhythmic gene expression at the core of the oscillators. In addition to the well-characterized transcriptional feedback regulation of several clock components, additional regulatory mechanisms, such as alternative splicing, regulation of protein stability, and chromatin modifications are beginning to emerge. In this review, we discuss recent findings in the regulation of the circadian clock function in Arabidopsis thaliana. The involvement of chromatin modifications in the regulation of the core circadian clock genes is also discussed. Keywords: circadian clock; oscillators; transcriptional and post-transcriptional regulation; chromatin modifications; Arabidopsis 1. Introduction Plants, like animals, exhibit rhythmic biological activity, with a periodicity of 24 h. -
![Plant Physiol 140:933-945 [Pdf]](https://docslib.b-cdn.net/cover/7281/plant-physiol-140-933-945-pdf-877281.webp)
Plant Physiol 140:933-945 [Pdf]
Forward Genetic Analysis of the Circadian Clock Separates the Multiple Functions of ZEITLUPE1[W] E´ va Kevei, Pe´ter Gyula, Anthony Hall, La´szlo´ Kozma-Bogna´r, Woe-Yeon Kim, Maria E. Eriksson, Re´ka To´th, Shigeru Hanano, Bala´zs Fehe´r, Megan M. Southern, Ruth M. Bastow, Andra´sViczia´n, Victoria Hibberd, Seth J. Davis, David E. Somers, Ferenc Nagy, and Andrew J. Millar* Institute of Plant Biology, Biological Research Centre of the Hungarian Academy of Sciences, H–6726 Szeged, Hungary (E´ .K., P.G., B.F., F.N.); School of Biological Sciences, University of Liverpool, Liverpool L69 7ZB, United Kingdom (A.H.); Institute of Molecular Plant Science, University of Edinburgh, Edinburgh EH9 3JH, United Kingdom (L.K.-B., A.J.M.); Department of Plant Cellular and Molecular Biology, Ohio State University, Columbus, Ohio 43210 (W.-Y.K., D.E.S.); Umea˚ Plant Science Centre, Department of Plant Physiology, Umea˚ University, SE–901 87 Umea, Sweden (M.E.E.); Department of Plant Developmental Biology, Max Planck Institute for Plant Breeding Research, D–50829 Cologne, Germany (R.T., S.H., S.J.D.); Department of Biological Sciences, University of Warwick, Coventry CV4 7AL, United Kingdom (M.M.S., R.M.B., V.H.); and Biologie II/Institut fu¨ r Botanik, University of Freiburg, D–79104 Freiburg, Germany (A.V.) The circadian system of Arabidopsis (Arabidopsis thaliana) includes feedback loops of gene regulation that generate 24-h oscillations. Components of these loops remain to be identified; none of the known components is completely understood, including ZEITLUPE (ZTL), a gene implicated in regulated protein degradation. -

How Plants Tell the Time Giovanni Murtas* and Andrew J Millar†
43 How plants tell the time Giovanni Murtas* and Andrew J Millar† The components of the circadian system that have recently match biological time with solar time. As light is an impor- been discovered in plants share some characteristics with tant environmental cue for the entrainment of the those from cyanobacterial, fungal and animal circadian clocks. circadian clock, a long-standing goal has been the identifi- Light input signals to the clock are contributed by multiple cation of the specific photoreceptors that are responsible photoreceptors: some of these have now been shown to for resetting the oscillator [2,3]. The Kay team [4••] has function specifically in response to light of defined wavelength now reported that the phytochromes A and B (phyA and and fluence rate. New reports of clock-controlled processes phyB), and cryptochrome 1 (cry1) are circadian input pho- and genes are highlighting the importance of time management toreceptors. They tested the circadian regulation of the for plant development. clock-responsive CHLOROPHYLL A/B-BINDING PRO- TEIN 2 (CAB2) promoter in Arabidopsis plants carrying Addresses photoreceptor mutations using the firefly luciferase Department of Biological Sciences, University of Warwick, Gibbet Hill reporter gene (luc). They found that the period of the Road, Coventry CV4 7AL, UK CAB2::luc activity rhythm is shortened under constant *e-mail: [email protected] light — a response that is mediated by the photoreceptor †e-mail: [email protected] classes that are sensitive to red and blue light [3]. Current Opinion in Plant Biology 2000, 3:43–46 Measurements of periodicity under a range of light inten- 1369-5266/00/$ — see front matter © 2000 Elsevier Science Ltd. -

Complex Interactions in the Arabidopsis Circadian Clock
bioRxiv preprint doi: https://doi.org/10.1101/068460; this version posted August 8, 2016. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 Title: Into the Evening: Complex Interactions in the Arabidopsis 2 circadian clock. 3 He Huang1 and Dmitri A. Nusinow1,* 4 1Donald Danforth Plant Science Center, St. Louis, MO 63132, USA 5 *Correspondence: [email protected] (D.A. Nusinow) 6 7 8 Key words: evening complex, clock, light, temperature, hypocotyl elongation, flowering 9 10 11 1 bioRxiv preprint doi: https://doi.org/10.1101/068460; this version posted August 8, 2016. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 12 Abstract 13 In Arabidopsis thaliana, an assembly of proteins named the evening complex (EC) has 14 been established as an essential component of the circadian clock with conserved 15 functions in regulating plant growth and development. Recent studies identifying EC- 16 regulated genes and EC-interacting proteins have expanded our understanding of EC 17 function. In this review, we focus on new progress uncovering how the EC contributes to 18 the circadian network through the integration of environmental inputs and the direct 19 regulation of key clock genes. We also summarize new findings of how the EC directly 20 regulates clock outputs, such as day-length dependent and thermoresponsive growth, 21 and provide new perspectives on future experiments to address unsolved questions 22 related to the EC. -

Diversification of the Core RNA Interference Machinery In
Copyright Ó 2008 by the Genetics Society of America DOI: 10.1534/genetics.107.086546 Diversification of the Core RNA Interference Machinery in Chlamydomonas reinhardtii and the Role of DCL1 in Transposon Silencing J. Armando Casas-Mollano,1 Jennifer Rohr,1 Eun-Jeong Kim, Eniko Balassa, Karin van Dijk2 and Heriberto Cerutti3 School of Biological Sciences and Plant Science Initiative, University of Nebraska, Lincoln, Nebraska 68588 Manuscript received December 28, 2007 Accepted for publication March 5, 2008 ABSTRACT Small RNA-guided gene silencing is an evolutionarily conserved process that operates by a variety of molecular mechanisms. In multicellular eukaryotes, the core components of RNA-mediated silencing have significantly expanded and diversified, resulting in partly distinct pathways for the epigenetic control of gene expression and genomic parasites. In contrast, many unicellular organisms with small nuclear genomes seem to have lost entirely the RNA-silencing machinery or have retained only a basic set of components. We report here that Chlamydomonas reinhardtii, a unicellular eukaryote with a relatively large nuclear genome, has undergone extensive duplication of Dicer and Argonaute polypeptides after the divergence of the green algae and land plant lineages. Chlamydomonas encodes three Dicers and three Argonautes with DICER- LIKE1 (DCL1) and ARGONAUTE1 being more divergent than the other paralogs. Interestingly, DCL1 is uniquely involved in the post-transcriptional silencing of retrotransposons such as TOC1. Moreover, on the basis of the subcellular distribution of TOC1 small RNAs and target transcripts, this pathway most likely operates in the nucleus. However, Chlamydomonas also relies on a DCL1-independent, transcriptional silencing mechanism(s) for the maintenance of transposon repression. -

Arabidopsis Circadian Clock Protein, TOC1, Is a DNA-Binding Transcription Factor
Arabidopsis circadian clock protein, TOC1, is a DNA-binding transcription factor Joshua M. Gendron, José L. Pruneda-Paz, Colleen J. Doherty, Andrew M. Gross, S. Earl Kang, and Steve A. Kay1 Section of Cell and Developmental Biology, Division of Biological Sciences, University of California at San Diego, La Jolla, CA 92093-0130 Contributed by Steve A. Kay, January 9, 2012 (sent for review January 5, 2012) The first described feedback loop of the Arabidopsis circadian in the C terminus (12). The PR domain allows homo- and hetero- clock is based on reciprocal regulation between TIMING OF CAB oligomerization between the PRRs and bridges interactions of the EXPRESSION 1 (TOC1) and CIRCADIAN CLOCK-ASSOCIATED 1 PRRs and other proteins (13–15). The CCT domain of CO, a di- (CCA1)/LATE ELONGATED HYPOCOTYL (LHY). CCA1 and LHY are rect transcriptional activator of FLOWERING LOCUS T, was Myb transcription factors that bind directly to the TOC1 promoter recently shown to bind DNA (16). This function has not been to negatively regulate its expression. Conversely, the activity of reported for the TOC1/PRR CCT domains, although TOC1 and TOC1 has remained less well characterized. Genetic data support the PRRs occupy regions of the CCA1/LHY promoter in ChIP that TOC1 is necessary for the reactivation of CCA1/LHY, but there experiments (17–19). It was shown for TOC1 and hypothesized is little description of its biochemical function. Here we show that for the PRRs that this association with chromatin could be TOC1 occupies specific genomic regions in the CCA1 and LHY pro- bridged by other DNA-binding factors, such as CCA1 Hiking moters. -

Download Full Text
This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and education use, including for instruction at the authors institution and sharing with colleagues. Other uses, including reproduction and distribution, or selling or licensing copies, or posting to personal, institutional or third party websites are prohibited. In most cases authors are permitted to post their version of the article (e.g. in Word or Tex form) to their personal website or institutional repository. Authors requiring further information regarding Elsevier’s archiving and manuscript policies are encouraged to visit: http://www.elsevier.com/copyright Author's personal copy Available online at www.sciencedirect.com Comes a time C Robertson McClung The circadian clock is a selfsustaining oscillator with an There was a time when a review of the current research in endogenous period of 24 hours. The Arabidopsis clock is plant circadian rhythms for Current Opinions could be composed of a set of interlocking negative feedback loops comprehensive. Comes a time when the volume of entailing transcriptional, post-transcriptional, and post- research simply exceeds the word limit of this type of translational, particularly regulated proteolysis, control. Clock review. Plant circadian research has soared past this control of the transcriptome is widespread; up to 90% of the threshold, which is tremendously exciting but necessi- transcriptome cycles in at least one condition in seedlings tates an apology to those whose work is omitted (but exposed to a variety of environmental cycles. Clock control certainly not overlooked). Comes a time also when the old extends to the metabolome, though diurnal oscillations in ways of gene-at-a-time analysis must be augmented with enzyme activities and metabolites are less dramatic than systems-level analysis. -
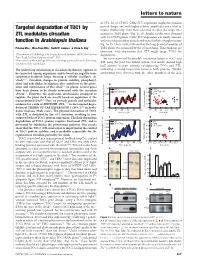
Targeted Degradation of TOC1 by ZTL Modulates Circadian Function In
letters to nature .............................................................. of ZTL. In ztl-1/TMG, CAB2::LUC expression displayed circadian periods longer and with higher relative amplitudes error (that is, Targeted degradation of TOC1 by weaker rhythmicity) than those observed in either the single ztl-1 ZTL modulates circadian mutant or TMG plants (Fig. 1c, d). Similar results were obtained with ztl-3/TMG plants; CAB2::LUC expression was clearly affected, function in Arabidopsis thaliana with very long circadian periods and weak rhythms (Supplementary Fig. 1a, b). These results indicate that the long-period phenotype of Paloma Ma´s1, Woe-Yeon Kim2, David E. Somers2 & Steve A. Kay1 TMG plants was enhanced by the ztl mutations. These findings are consistentwiththenotionthatZTLmighttargetTOC1for 1Department of Cell Biology, The Scripps Research Institute, 10550 North Torrey degradation. Pines Road, La Jolla, California 92037, USA 2 We next examined the possible interaction between TOC1 and Department of Plant Biology, Plant Biotechnology Center, Ohio State University, ZTL using the yeast two-hybrid system. Our results showed high Columbus, Ohio 43210, USA ............................................................................................................................................................................. lacZ activity in yeast colonies co-expressing TOC1 and ZTL, The underlying mechanism of circadian rhythmicity appears to indicating a strong interaction between both proteins. Weaker be conserved among organisms, and is based on negative tran- interactions were detected with the other members of the ZTL scriptional feedback loops forming a cellular oscillator (or ‘clock’)1,2. Circadian changes in protein stability, phosphoryl- ation and subcellular localization also contribute to the gener- ation and maintenance of this clock1,2. In plants, several genes have been shown to be closely associated with the circadian system3,4. -

TCP Transcription Factors: Architectures of Plant Form
DOI 10.1515/bmc-2012-0051 BioMol Concepts 2013; 4(2): 111–127 Review Nora G. Uberti Manassero , Ivana L. Viola , Elina Welchen and Daniel H. Gonzalez * TCP transcription factors: architectures of plant form Abstract: After its initial definition in 1999, the TCP fam- Introduction ily of transcription factors has become the focus of a multiplicity of studies related with plant development at TCP proteins (TCPs) are plant transcription factors the cellular, organ, and tissue levels. Evidence has accu- involved in diverse growth-related processes such as mulated indicating that TCP transcription factors are the embryonic growth, leaf development, branching, floral main regulators of plant form and architecture and con- organ morphogenesis, pollen development, germina- stitute a tool through which evolution shapes plant diver- tion, senescence, circadian rhythm, cell cycle regula- sity. The TCP transcription factors act in a multiplicity tion, and hormone signaling (1) . These proteins contain of pathways related with cell proliferation and hormone a highly conserved domain, the TCP domain, defined by responses. In recent years, the molecular pathways of TCP the first identified members of the family: TB1 (teosinte protein action and biochemical studies on their mode of branched1), CYC (cycloidea), and PCF1 and 2 (2) . The TCP interaction with DNA have begun to shed light on their domain is involved in DNA binding and dimerization. Sec- mechanism of action. However, the available information ondary structure predictions suggest that the TCP domain is fragmented and a unifying view of TCP protein action is is formed by an N-terminal region enriched in basic amino lacking, as well as detailed structural studies of the TCP- acids followed by two amphipathic α -helices connected DNA complex. -

1501.Full.Pdf
Copyright Ó 2007 by the Genetics Society of America DOI: 10.1534/genetics.107.072769 A Complex Genetic Interaction Between Arabidopsis thaliana TOC1 and CCA1/LHY in Driving the Circadian Clock and in Output Regulation Zhaojun Ding,* Mark R. Doyle,† Richard M. Amasino† and Seth J. Davis*,1 *Max Planck Institute for Plant Breeding Research, D-50829 Cologne, Germany and †Department of Biochemistry, University of Wisconsin, Madison, Wisconsin 53706-1544 Manuscript received March 1, 2007 Accepted for publication April 30, 2007 ABSTRACT It has been proposed that CIRCADIAN CLOCK ASSOCIATED 1 (CCA1) and LATE ELONGATED HYPOCOTYL (LHY) together with TIMING OF CAB EXPRESSION 1 (TOC1) make up the central oscillator of the Arabidopsis thaliana circadian clock. These genes thus drive rhythmic outputs, including seasonal control of flowering and photomorphogenesis. To test various clock models and to disclose the genetic relationship between TOC1 and CCA1/LHY in floral induction and photomorphogenesis, we constructed the cca1 lhy toc1 triple mutant and cca1 toc1 and lhy toc1 double mutants and tested various rhythmic responses and circadian output regulation. Here we report that rhythmic activity was dra- matically attenuated in cca1 lhy toc1. Interestingly, we also found that TOC1 regulates the floral transition in a CCA1/LHY-dependent manner while CCA1/LHY functions upstream of TOC1 in regulating a pho- tomorphogenic process. This suggests to us that TOC1 and CCA1/LHY participate in these two processes through different strategies. Collectively, we have used genetics to provide direct experimental support of previous modeling efforts where CCA1/LHY, along with TOC1, drives the circadian oscillator and have shown that this clock is essential for correct output regulation. -

ELF4 Is a Phytochrome-Regulated Component of a Negative-Feedback
The Plant Journal (2005) 44, 300–313 doi: 10.1111/j.1365-313X.2005.02531.x ELF4 is a phytochrome-regulated component of a negative- feedback loop involving the central oscillator components CCA1 and LHY Elise A. Kikis1,2, Rajnish Khanna1,2 and Peter H. Quail1,2,* 1Department of Plant and Microbial Biology, University of California, Berkeley, CA 94720, USA, and 2USDA/ARS – Plant Gene Expression Center, 800 Buchanan Street, Albany, CA 94710, USA Received 20 May 2005; revised 19 July 2005; accepted 29 July 2005. *For correspondence (fax þ0 510 559 5678; e-mail [email protected]). Summary Evidence has been presented that a negative transcriptional feedback loop formed by the genes CIRCADIAN CLOCK ASSOCIATED (CCA1), LATE ELONGATED HYPOCOTYL (LHY) and TIMING OF CAB (TOC1) constitutes the core of the central oscillator of the circadian clock in Arabidopsis. Here we show that these genes are expressed at constant, basal levels in dark-grown seedlings. Transfer to constant red light (Rc) rapidly induces a biphasic pattern of CCA1 and LHY expression, and a reciprocal TOC1 expression pattern over the first 24 h, consistent with initial induction of this synchronous oscillation by the light signal. We have used this assay with wild-type and mutant seedlings to examine the role of these oscillator components, and to determine the function of ELF3 and ELF4 in their light-regulated expression. The data show that whereas TOC1 is necessary for light-induced CCA1/LHY expression, the combined absence of CCA1 and LHY has little effect on the pattern of light-induced TOC1 expression, indicating that the negative regulatory arm of the proposed oscillator is not fully functional during initial seedling de-etiolation.