Richness, Systematics, and Distribution of Molluscs Associated
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Oup Mollus Eyx029 384..398 ++
Journal of The Malacological Society of London Molluscan Studies Journal of Molluscan Studies (2017) 83: 384–398. doi:10.1093/mollus/eyx029 Advance Access publication date: 17 July 2017 Featured Article One for each ocean: revision of the Bursa granularis (Röding, 1798) species complex (Gastropoda: Tonnoidea: Bursidae) Downloaded from https://academic.oup.com/mollus/article-abstract/83/4/384/3977763 by IFREMER user on 25 January 2019 Malcolm T. Sanders1,2, Didier Merle1, Philippe Bouchet2, Magalie Castelin2, Alan G. Beu3, Sarah Samadi2 and Nicolas Puillandre2 1Centre de Recherche sur la Paléobiodiversité et les Paléoenvironnements CR2P – UMR7207 – CNRS, MNHN, UPMC, Muséum national d’Histoire naturelle, Sorbonne Universités, 8 rue Buffon, CP 38, 75005 Paris, France; 2Institut de Systématique, Évolution, Biodiversité ISYEB – UMR 7205 – CNRS, MNHN, UPMC, EPHE, Muséum national d’Histoire naturelle, Sorbonne Universités, 57 rue Cuvier, CP26, F-75005 Paris, France; and 3GNS Science, PO Box 30-368, Lower Hutt 5040, New Zealand Correspondence: N. Puillandre; e-mail: [email protected] (Received 20 March 2017; editorial decision 12 June 2017) ABSTRACT Bursa granularis (Röding, 1798) is a tonnoidean gastropod that is regarded as broadly distributed throughout the Indo-Pacific and tropical western Atlantic. Because of its variable shell it has received no less than thir- teen names, now all synonymized under the name B. granularis. We sequenced a fragment of the cox1 gene for 82 specimens covering a large part of its distribution and most type localities. Two delimitation meth- ods were applied, one based on genetic distance (ABGD) and one based on phylogenetic trees (GMYC). All analyses suggest that specimens identified as B. -

Spinucella a New Genus of Miocene to Pleistocene , Muricid Gastropods from the Eastern Atlantic
Contr. Tert. Quatern. Geol. 30(1-2) 19-27 1 tab., 1 pi. Leiden, June 1993 Spinucella a new genus of Miocene to Pleistocene , muricid gastropods from the eastern Atlantic Geerat J. Vermeij University of California Davis, U.S.A. — new of Miocene Pleistocene muricid from the Atlantic. Contr. Tert. Vermeij, GeeratJ. Spinucella, a genus to gastropods eastern Quatern. Geol., 30(1-2): 19-27, 1 tab., 1 pi. Leiden, June 1993. The muricid is for de C. 1825 from the Pliocene of the new gastropod genus Spinucella proposed Purpura tetragonaJ. Sowerby, (type species), North Sea Basin, and for several other early Miocene to late Pleistocene species from southern Europe, North Africa, and southern Africa. The is characterised the of labral on the of the shell and reticulate of genus by presence a spine outer lip by sculpture. Species and Acanthinucella Cooke, 1918. The Spinucella closely resemble members ofNucella Röding, 1798, Acanthina Fischer von Waldheim, 1807, ofthat in the eastern Pacific Acanthina andAcanthinucella. Withthe removal of labral spine of Spinucellawas probably evolved independendy from where authors have the the time of arrival of Nucella in the North Atlantic from the S. tetragona Nucella, many recent placed species, North Pacific was late Pliocene, rather than middle Pliocene. — words Key Spinucella, new genus, Acanthina, Nucella, Neogene, biogeography. Prof. Dr G.J. Vermeij, Department of Geology, University of California, Davis, CA 95616, U.S.A. Contents 1956; Glibert, 1959, 1963). In fact, Glibert (1959) considered N. tetragona to be ancestral to N. lapillus, Introduction 19 p. the two species being linked by the late Pliocene 20 Systematics p. -

Potencialidad De Puerto Deseado Y Camarones (Argentina)
46 Potencialidad de Puerto Deseado y Camarones (Argentina) como destinos de cruceros a partir de sus competencias territoriales Potentiality of Puerto Deseado and Camarones (Argentina) as cruise destinations from their territorial competences Historial del artículo Carolina Cohena, Marisol Veredab y Graciela Bensenyc Recibido: 26 de mayo de 2020 a Universidad Nacional de Tierra del Fuego, Antártida e Islas del Atlántico Sur, Ushuaia, Argentina. Beca Postdoctoral CONICET. Revisado Correo electrónico: [email protected] 19 de octubre de 2020 b Universidad Nacional de Tierra del Fuego, Antártida e Islas del Atlántico Sur, Ushuaia, Argentina. Aceptado: c Universidad Nacional de Mar del Plata, Mar del Plata, Argentina. 03 de noviembre de 2020 Palabras clave Resumen Competitividad, Patagonia El turismo de cruceros está comúnmente asociado a una práctica exógenamente determinada. Sin embargo, como argentina, Puerto Deseado práctica social, se emplaza en un espacio cargado de connotaciones sociales y culturales; haciendo uso de un territorio y Camarones, turismo de construido socialmente. Desde esta mirada la decisión de optar por este tipo de actividad es tomada teniendo en cruceros cuenta un conjunto de elementos que va más allá de las características del buque. Los territorios en cuestión deben poseer una serie de condiciones que resulten favorables para su competitividad, fundamentalmente asociadas a la presencia de recursos naturales y culturales que conforman la oferta de sitios potenciales de visita y la prestación de servicios. Estos factores forman parte de las competencias territoriales que poseen los destinos y pueden actuar como elementos que favorezcan y potencien su inserción en el turismo de cruceros. Puerto Deseado y Camarones, dos puertos de la Patagonia argentina, han mostrado interés por esta actividad surgiendo el interrogante respecto a las competencias territoriales que poseen para el desarrollo del turismo de cruceros. -

Darwin's Route from Ushuaia
Darwin’s Route from Ushuaia Departing from Ushuaia, retrace the route of Charles Darwin aboard HMS Beagle on an expedition cruise through the secluded Fuegian Archipelago at the bottom of South America. Our adventurous nine-day (eight-night) itinerary includes legendary Cape Horn and historic Wulaia Bay, as well as Glacier Alley, the penguin boisterous colonies on Tuckers and Magdalena islands, as well as the spectacular fjords that harbor Pía and Águila glaciers. While visiting Patagonia you'll also encounter massive ice fields, lush sub-polar forests and secluded beaches on islands that remain refreshingly remote and barely touched by civilization, a rare glimpse of what planet Earth must have been like before mankind. Midway through the journey, a half- day port call in Punta Arenas leaves plenty of time to explore a city rich in history, architecture and Patagonian culture before resuming the journey back to Ushuaia. (B = Breakfast, L = Lunch, D = Dinner) NOTE: You can also start in Punta Arenas. Similar itinerary available on the Stella Australis. Operates between September & April. Please ask for more details. Day 1: Ushuaia Check in at 409 San Martín Ave. in downtown Ushuaia between 10:00 and 17:00 (10 AM-5 PM) on the day of your cruise departure. 2016-2017 Season: Board the M/V Stella Australis at 17:30 (5:30 PM). 2017-2018 Season: Board the M/V Stella Australis at 18:00 (6 PM). After a welcoming toast and introduction of captain and crew, the ship departs for one of the most remote corners of planet Earth. -

Geochemical Characteristics of the Infilling of Ground Wedges at Puerto Deseado (Santa Cruz, Argentina): Palaeoenvironmental and Chronological Implications
Andean Geology ISSN: 0718-7092 ISSN: 0718-7106 [email protected] Servicio Nacional de Geología y Minería Chile Geochemical characteristics of the infilling of ground wedges at Puerto Deseado (Santa Cruz, Argentina): palaeoenvironmental and chronological implications Zanchetta, Giovanni; Ribolini, Adriano; Ferrari, Matteo; Bini, Monica; Isola, Ilaria; Lezzerini, Marco; Baroni, Carlo; Salvatore, Maria Cristina; Pappalardo, Marta; Fucks, Enrique; Boretto, Gabriella Geochemical characteristics of the infilling of ground wedges at Puerto Deseado (Santa Cruz, Argentina): palaeoenvironmental and chronological implications Andean Geology, vol. 45, no. 2, 2018 Servicio Nacional de Geología y Minería, Chile Available in: https://www.redalyc.org/articulo.oa?id=173955659002 DOI: https://doi.org/10.5027/andgeoV45n2-3070 PDF generated from XML JATS4R by Redalyc Project academic non-profit, developed under the open access initiative Andean Geology, 2018, vol. 45, no. 2, ISSN: 0718-7092 0718-7106 Artículos de Investigación Geochemical characteristics of the infilling of ground wedges at Puerto Deseado (Santa Cruz, Argentina): palaeoenvironmental and chronological implications Características geoquímicas de las cuñas de hielo en Puerto Deseado (Santa Cruz, Argentina): implicancias paleoambientales y cronológicas Giovanni Zanchetta DOI: https://doi.org/10.5027/andgeoV45n2-3070 Istituto Nazionale Geofisica e Vulcanologia Roma, Italia Redalyc: https://www.redalyc.org/articulo.oa? [email protected] id=173955659002 Adriano Ribolini University -

Patagonian Explorer
PATAGONIAN EXPLORER Explore Patagonia on an adventurous five-day, four-night journeys between Ushuaia (Argentina) to Punta Arenas (Chile) through some of planet’s most remote places and incredible scenery. Discover the wild beauty of Patagonia on an Australis cruise that showcases the region’s pristine landscapes, rich wildlife and fascinating human history aboard an expedition ship that brings an extraordinary level of comfort and service to the uttermost edge of the world. Leaving the twinkling lights of Ushuaia behind, the vessel calls on fabled spots like Cape Horn and Wulaia Bay before cruising down the Beagle Channel. Continuing through the maze of islands, we’ll visit a number of glaciers, frozen giants that guard the southern flank of the ITINERARY Fuegian Archipelago, before entering the legendary Strait of Day 1 - Ushuaia Magellan. Last stop is the Isla Magdalena and its boisterous Check in at 160 Juan Manuel de Rosas Street in downtown Patagonia penguin colony before docking at Punta Arenas. Ushuaia between 10:00 and 17:00 (10 AM-5 PM) on the day of your cruise departure. Board the M/V Ventus Australis at 18:00 (6 PM). After a welcoming toast and introduction of captain and crew, the ship departs for one of the most remote corners of planet Earth. During the night we traverse the Beagle Channel and cross from Argentina into Chilean territorial waters. The lights of Ushuaia disappear as we turn into the narrow Murray Channel between Navarino and Hoste islands. Day 2 - Cape Horn - Wulaia Bay Around the break of dawn, Stella Australis crosses Nassau Bay and enters the remote archipelago that comprises Cape Horn National Park. -

The Marine Fauna of New Zealand: the Molluscan Genera Cymatona and Fusitriton (Gastropoda, Family Cymatiidae)
ISSN 0083-7903, 65 (Print) ISSN 2538-1016; 65 (Online) The Marine Fauna of New Zealand: The Molluscan Genera Cymatona and Fusitriton (Gastropoda, Family Cymatiidae) by A. G. BEU New Zealand Oceanographic Institute Memoir 65 1978 NEW ZEALAND DEPARTMENT OF SCIENTIFIC AND INDUSTRIAL RESEARCH The Marine Fauna of New Zealand: The Molluscan Genera Cymatona and Fusitriton (Gastropoda, Family Cymatiidae) by A. G. BEU New Zealand Geological Survey, DSIR, Lower Hutt New Zealand Oceanographic Institute Memoir 65 This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/ Citation according to ''World List of Scientific Periodicals" (4th edn.): Mem. N.Z. oceanogr. Inst. 65 Received for publication September 1973 © Crown Copyright 1978 This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/ CONTENTS Page Abstract . � 5 INTRODUCTION 5 4AXONOMY 10 Family CYMATIIDAE 10 Genus Cymatona 10 Cymatona kampyla 10 Cymatona kampyla kampyla 12 Cymatona kampyla tomlini . 18 Cymatona kampyla jobbernsi 18 Genus Fusitriton 18 Fusitriton cancellatus 22 Fusitriton cancellatus retiolus 22 Fusitriton cance/latus laudandus 23 ECOLOGY . 25 Benthic sampling programme of N.Z. Oceanographic Institute 25 Sampling methods 25 Distribution anomalies 25 Distribution 26 Distribution with depth 26 Distribution with latitude 27 Distribution with sediment type 27 Ecological conclusions 33 Dispersal times and routes of Fusitriton, and their effect on Cymatona 34 Dispersal and distribution 34 Ecological displacement of Cymatona kampyla kampyla 35 ACKNOWLEDGMENTS 36 REFERENCES 36 APPENDIX 1: Station List 38 APPENDIX 2: Dimensions of Cymatona 41 APPENDIX 3: Dimensions of Fusitriton 42 INDEX 44 This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. -

Day 1: Punta Arenas Check in at 1385 O'higgins Street (Arturo Prat Port) in Punta Arenas Between 13:00 and 17:00 (1-5 PM) on T
Day 1: Punta Arenas Check in at 1385 O’Higgins Street (Arturo Prat Port) in Punta Arenas between 13:00 and 17:00 (1-5 PM) on the day of your cruise departure. Board the M/V Stella Australis or M/V Via Australis at 18:00 (6 PM). After a welcoming cocktail reception hosted by the captain and his crew, the ship departs for one of the remotest corners of planet Earth. During the night we cross the Strait of Magellan and enter the labyrinth of channels that define the southern extreme of Patagonian. The twinkling lights of Punta Arenas gradually fade into the distance as we enter the Whiteside Canal between Darwin Island and Isla Grande de Tierra del Fuego. Day 2: Ainsworth Bay – Tuckers Islets By dawn the ship is sailing up Admiralty Sound, a spectacular offshoot of the Strait of Magellan that stretches nearly halfway across Tierra del Fuego. The snow-capped peaks of Karukinka Natural Park stretch along the north side of the sound, while the south shore is defined by the deep fjords and broad bays of Alberto de Agostini National Park. We go ashore at Ainsworth Bay, which harbours copious bird life and a colony of southern elephant seals which can sometimes be spotted from the Zodiacs. Two guided excursions are available: one is along the edge of a stream, peat bog and beaver habitat to a waterfall-and-moss-covered rock face tucked deep inside a pristine sub-polar forest; the other is a more strenuous hike along the crest of a glacial moraine. -

Uplift of Quaternary Shorelines in Eastern Patagonia: Darwin Revisited
Uplift of Quaternary shorelines in Eastern Patagonia: Darwin revisited Kevin Pedoja, Vincent Regard, Laurent Husson, Joseph Martinod, Benjamin Guillaume, Enrique Fucks, Maximiliano Iglesias, Pierre Weill To cite this version: Kevin Pedoja, Vincent Regard, Laurent Husson, Joseph Martinod, Benjamin Guillaume, et al.. Uplift of Quaternary shorelines in Eastern Patagonia: Darwin revisited. Geomorphology, Elsevier, 2011, 127 (3-4), pp.121-142. 10.1016/j.geomorph.2010.08.003. insu-00610899 HAL Id: insu-00610899 https://hal-insu.archives-ouvertes.fr/insu-00610899 Submitted on 6 Mar 2019 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Distributed under a Creative Commons Attribution| 4.0 International License Uplift of quaternary shorelines in eastern Patagonia: Darwin revisited Kevin Pedoja a,⁎, Vincent Regard b,c,d, Laurent Husson e,f, Joseph Martinod b,c,d, Benjamin Guillaume g, Enrique Fucks h, Maximiliano Iglesias i, Pierre Weill a a Laboratoire de Morphodynamique Continentale et Côtière, CNRS, Université de Caen, 14000 Caen, France b Université de Toulouse ; UPS (OMP) ; LMTG ; 14 Av Edouard Belin, F-31400 Toulouse, France c IRD ; LMTG ; F-31400 Toulouse, France d CNRS ; LMTG ; F-31400 Toulouse, France e CNRS UMR 6118, Géosciences Rennes, 35042 Rennes, France f CNRS UMR 6112, Laboratoire de Planétologie et Géodynamique de Nantes, France g Dip. -

Wap Directory 2021-LH.Pdf
SECTION 3 Lighthouses (Beacon, Headlight, Balizas, Radar Reflectors) in Antarctica & Peri Antarctic areas (Reference sites: https://en.wikipedia.org/wiki/List_of_lighthouses_in_Antarctica ; https://www.unc.edu/~rowlett/lighthouse/ata.htm; SUB & PERI-ANTARCTIC TERRITORIES (Included Localities among 6Ø° and 53° Southern parallels of Austral hemisphere, plus few other Territories selected according to the Peri Antarctic Islands map attached.) AUSTRAL TERRITORIES (Included only Territories here selected among 37°.5Ø’ and 53° South of Austral hemisphere.) W.A.P. Base, Camp, Hut, Refuge, Station WADA Location Longitude Callsigns Date Name Ref. Latitude ARGENTINA ARG-LH-ØØ1 Baliza Potter (aka: ARLHS: SSI ØØ3; G.1387.7; NGIA: 111-2725) Potter Bay, Bay of Guardia Nacional, King George Island 62°14'16"S 58°39'52"W LU1ZI 2Ø11 ARG-LH-ØØ2 Baliza CÁmara -Tres Hermanos LH (aka: ARLHS: SSI ØØ4; G.1387.6; NGIA: 111-2724) Potter Cove, King George Island 62°14'24"S 58°4Ø'42"W LU4AA/Z 2Ø16,17-19/Ø2/2Ø17 ARG-LH-ØØ4 Baliza Punta Páramo (aka: ARLHS ARG Ø57, G.1261; NGIA: 11Ø-2Ø16Ø) Península El Páramo, San Sebastian Bay, Tierra del Fuego 53°Ø9'ØØ"S 68°13'ØØ"W LU2XX ??? ARG-LH-ØØ5 Les Eclarieur LH (aka: ARLHS: ARG Ø16; G.132Ø; NGIA: 111-262Ø) Les Eclarieur Island, Tierra del Fuego 54°52'18"S 68°Ø5'ØØ"W LU8XW/P2Ø14 ARG-LH-ØØ6 Baliza Punta Observatorio (aka: ARG-Ø8Ø ex AR-ØØ49, G1235, LH2448) Tierra del Fuego 54°49'ØØ"S 68°18'ØØ"W LT5X 2Ø11 ARG-LH-ØØ7 Faro San Juan de Salvamento (aka: ARG-ØØ2, G1283) Isla de lo s Estados. -
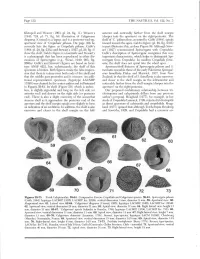
(1943: 724, Pi. 71, Fig. 16) Illustration of Calyptraea (Deeper Into the Aperture) on the Right/Posterior
Page 118 THE NAUTILUS, Vol. 122, No. 3 Kleinpeil and Weaver (1963, pi. 24, fig. 11). Weavers anterior and noticeably farther from the shell margin (1943: 724, pi. 71, fig. 16) illustration of Calyptraea (deeper into the aperture) on the right/posterior. The diegoana (Conrad) is a lapsus and is a posterior-end-up, shelf of 'C.y pileum thus, as noted by Gabb (1864), spirals apertural view of 'Crepidula' pileum. On page 356 he inward toward the apex. Gabb's figure (pi. 29, fig. 233b) correctly lists the figure as Crepidula pileum. Gabb's in part illustrates this, as does Figure 59. Although Stew- (1864: pi. 29, fig. 233a) and Stewart's (1927, pi. 29, fig. 3) art (1927) synonomized Spirocrypta with Crepidula, show the shelf. Gabb's figure is a fascimile and Stewart's Gabb's description of Spirocrypta recognizes this very is a photograph that has been reproduced in other dis- important characteristic, which helps to distinguish Spi- cussions of Spirocrypta (e.g., Wenz, 1940: 903, fig. rocrypta from Crepidula. In modern Crepidula forni- 2660a). Gabb's and Stewart's figures are based on lecto- cata, the shelf does not spiral into the whorl apex. type ANSP 4221, but, unfortunately, the shelf of this Aperture/shelf features of Spirocrypta pileum and S. specimen is broken. Both figures create the false impres- inomata resemble those of the early Paleocene Spirogal- sion that there is a sinus near both ends of the shelf and erus lamellaria Finlay and Marwick, 1937, from New that the middle part protrudes and is concave. -

La Expedición De Los Hermanos Nodal Y Diego Ramírez De Arellano. El Legado En La Cartografía Hispana Del Siglo Xvii
MAGALLANIA (Chile), 2020. Volumen especial. El viaje de Magallanes, 1520-2020: 79-97 103 LA EXPEDICIÓN DE LOS HERMANOS NODAL Y DIEGO RAMÍREZ DE ARELLANO. EL LEGADO EN LA CARTOGRAFÍA HISPANA DEL SIGLO XVII RODRIGO MORENO J.a & FRANCISCA RODRÍGUEZ B.b RESUMEN Hace cuatro siglos los hermanos Bartolomé y Gonzalo Nodal, junto con el piloto Diego Ramírez de Arellano, fueron enviados por la corona hispana a los mares australes de América con la misión de verificar el hallazgo neerlandés del estrecho Le Maire y el cabo de Hornos. Tras corroborar los referidos hitos geográficos, rebautizándolos como estrecho de San Vicente y cabo de San Ildefonso, recorrieron el territorio descubriendo las islas Diego Ramírez, posteriormente regresando a España por la vía del estrecho de Magallanes en 1619. Las consecuencias de esta expedición no tuvieron el impacto esperado en la cartografía impresa europea, a excepción del referido archipiélago descubierto y otros topónimos menores, pero sí tuvieron influencia en la cartografía y la navegación española del siglo XVII, en particular en los derroteros náuticos manuscritos que se utilizaron en el Mar del Sur. PALABRAS CLAVE: Bartolomé y Gonzalo Nodal, Diego Ramírez de Arellano, estrecho Le Maire, cabo de Hornos, cartografía. THE EXPEDITION OF THE BROTHERS NODAL AND DIEGO RAMÍREZ DE ARELLANO. THE LEGACY IN HISPANIC CARTOGRAPHY OF THE 17TH CENTURY ABSTRACT Four centuries ago the brothers Bartolomé and Gonzalo Nodal, together with the pilot Diego Ramírez de Arellano, were sent by the Spanish crown to the southern seas of America with the mission of verifying the Dutch discovery of the Le Maire Strait and Cape Horn.