Atcveljavni 2014.Xlsx
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Classification of Medicinal Drugs and Driving: Co-Ordination and Synthesis Report
Project No. TREN-05-FP6TR-S07.61320-518404-DRUID DRUID Driving under the Influence of Drugs, Alcohol and Medicines Integrated Project 1.6. Sustainable Development, Global Change and Ecosystem 1.6.2: Sustainable Surface Transport 6th Framework Programme Deliverable 4.4.1 Classification of medicinal drugs and driving: Co-ordination and synthesis report. Due date of deliverable: 21.07.2011 Actual submission date: 21.07.2011 Revision date: 21.07.2011 Start date of project: 15.10.2006 Duration: 48 months Organisation name of lead contractor for this deliverable: UVA Revision 0.0 Project co-funded by the European Commission within the Sixth Framework Programme (2002-2006) Dissemination Level PU Public PP Restricted to other programme participants (including the Commission x Services) RE Restricted to a group specified by the consortium (including the Commission Services) CO Confidential, only for members of the consortium (including the Commission Services) DRUID 6th Framework Programme Deliverable D.4.4.1 Classification of medicinal drugs and driving: Co-ordination and synthesis report. Page 1 of 243 Classification of medicinal drugs and driving: Co-ordination and synthesis report. Authors Trinidad Gómez-Talegón, Inmaculada Fierro, M. Carmen Del Río, F. Javier Álvarez (UVa, University of Valladolid, Spain) Partners - Silvia Ravera, Susana Monteiro, Han de Gier (RUGPha, University of Groningen, the Netherlands) - Gertrude Van der Linden, Sara-Ann Legrand, Kristof Pil, Alain Verstraete (UGent, Ghent University, Belgium) - Michel Mallaret, Charles Mercier-Guyon, Isabelle Mercier-Guyon (UGren, University of Grenoble, Centre Regional de Pharmacovigilance, France) - Katerina Touliou (CERT-HIT, Centre for Research and Technology Hellas, Greece) - Michael Hei βing (BASt, Bundesanstalt für Straßenwesen, Germany). -

Cyproterone Art. 31
Приложение I Списък на лекарствените продукти и форми 1 Държава членка Притежател на Наименование на INN/Активно Фармацевтична Начин на (ЕИП) разрешението за продукта вещество + форма приложение употреба Количество на активното вещество (в дозова единица) Австрия Bayer Austria Gmbh Androcur Depot Cyproterone Acetate Инжекционен Интрамускулно 300mg/3ml разтвор приложение Австрия Bayer Austria Gmbh Climen Cyproterone Acetate 1mg Обвита таблетка Перорално таблетка, Estradiol приложение Valerate 2mg таблетка| Estradiol Valerate 2mg таблетка Австрия Bayer Austria Gmbh Climen 28-Tage Cyproterone Acetate 1mg Обвита таблетка Перорално таблетка, Estradiol приложение Valerate 2mg таблетка| Estradiol Valerate 2mg таблетка Австрия Bayer Austria Gmbh Diane Mite Cyproterone Acetate 2mg Обвита таблетка Перорално таблетка, Ethinylestradiol приложение 35μg таблетка Австрия Bayer Austria Gmbh Minerva Cyproterone Acetate 2mg Обвита таблетка Перорално таблетка, Ethinylestradiol приложение 0,035mg таблетка Австрия Bayer Austria Gmbh Andro-Diane Cyproterone Acetate Таблетка Перорално 10mg таблетка приложение Австрия Bayer Austria Gmbh Androcur Cyproterone Acetate Таблетка Перорално 100mg таблетка приложение Австрия Bayer Austria Gmbh Androcur Cyproterone Acetate Таблетка Перорално 50mg таблетка приложение Австрия Gynial Gmbh Alisma Cyproterone Acetate 2mg Филмирана таблетка Перорално таблетка, Ethinylestradiol приложение 35μg таблетка 2 Държава членка Притежател на Наименование на INN/Активно Фармацевтична Начин на (ЕИП) разрешението за продукта вещество + -

Aldosterone Enhances Renal Calcium Reabsorption by Two Types of Channels
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Kidney International, Vol. 66 (2004), pp. 242–250 Aldosterone enhances renal calcium reabsorption by two types of channels MARIE LECLERC,MICHELE` G. BRUNETTE, and DENIS COUCHOUREL Maisonneuve-Rosemont Hospital and University of Montreal, Canada Aldosterone enhances renal calcium reabsorption by two types transepithelial potential difference in the late segments of channels. of the nephron, reflecting a stimulation of the amiloride- Background. Aldosterone has been known for many years + + sensitive Na transport by the apical membranes. to increase sodium (Na ) reabsorption by the distal nephron. The present in vitro experiments investigated the effect of the In contrast to the abundant literature dealing with the + hormone on calcium (Ca2 ) transport by the luminal membrane antinatriuretic effect of aldosterone, data concerning its + of the rabbit nephron, independent of any systemic influence. action on Ca2 transport are relatively scarce. Clearance Methods. Proximal and distal tubules were incubated with studies showed either an increase in calciuria [2] or an + either aldosterone or the carrier. The luminal membranes of absence of significant change of Ca2 excretion [3, 4] af- these tubules were purified, vesiculated, and 45Ca uptake by these vesicles was subsequently measured. ter aldosterone administration. In a randomized study in Results. Treatment of the distal tubules with 10−8 mol/L al- male volunteers, Van Hamersvelt et al [5] compared the + + dosterone enhanced both 0.1 and 0.5 mmol/L Ca2 transport. effect of the Ca2 channel blocker felodipine, alone or The hormone action was abolished by tyrosine kinase inhibitors. -

Effect of Mgo Additive on Volumetric Expansion of Self-Degradable
Effect of MgO Additive on Volumetric Expansion ofSelf-degradable Cements Prepared for The U.S. Department of Energy Energy Efficiency and Renewable Energy Geothermal Technologies Program 1000 Independence Avenue SW Washington, D.C. 20585 Prepared hy Toshifumi Sugama, John Warren, and Thomas Butcher Sustainable Energy Technologies Department Brookhaven National Laboratory Upton, NY 11973-5000 Septemher 2011 Notice: This manuscript has been authored by employee of Brookhaven Science Associates, LLC under Contract No. DE-AC02-98CH t0886 with the U.S. Department of Energy. The publisher by accepting the manuscript for publication acknowledges that the United States Government retains a non.exclusjv~ paid-up, irrevocable, world·wide license to publish or reproduce the published form of this manuscript, or allow others to do so, for the United States Government purposes. DISCLAIMER This work was prepared as an account of work sponsored by an agency of the United States Government. Neither the United States Government nor any agency thereof, nor any of their employees, nor any of their contractors, subcontractors or their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or any third party’s use or the results of such use of any information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process, or service by trade name, trademark, manufacturer, or otherwise, does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States Government or any agency thereof or its contractors or subcontractors. -

The Conversion of Wollastonite to Caco3 Considering Its Use for CCS Application As Cementitious Material
applied sciences Article The Conversion of Wollastonite to CaCO3 Considering Its Use for CCS Application as Cementitious Material Kristoff Svensson *, Andreas Neumann, Flora Feitosa Menezes, Christof Lempp and Herbert Pöllmann Institute for Geosciences and Geography, Martin-Luther-University of Halle-Wittenberg, Von-Seckendorff-Platz 3, 06120 Halle (Saale), Germany; [email protected] (A.N.); fl[email protected] (F.F.M.); [email protected] (C.L.); [email protected] (H.P.) * Correspondence: [email protected]; Tel.: +49-345-55-26138 Received: 21 December 2017; Accepted: 8 February 2018; Published: 20 February 2018 Featured Application: Building materials and CCS. Abstract: The reaction of wollastonite (CaSiO3) with CO2 in the presence of aqueous solutions (H2O) and varied temperature conditions (296 K, 323 K, and 333 K) was investigated. The educts (CaSiO3) and the products (CaCO3 and SiO2) were analyzed by scanning electron microscopy (SEM), powder X-ray diffraction (PXRD), and differential scanning calorimetry with thermogravimetry coupled with a mass spectrometer and infrared spectrometer (DSC-TG/MS/IR). The reaction rate increased significantly at higher temperatures and seemed less dependent on applied pressure. It could be shown that under the defined conditions wollastonite can be applied as a cementitious material for sealing wells considering CCS applications, because after 24 h the degree of conversion from CaSiO3 to CaCO3 at 333 K was very high (>90%). As anticipated, the most likely application of wollastonite as a cementitious material in CCS would be for sealing the well after injection of CO2 in the reservoir. -
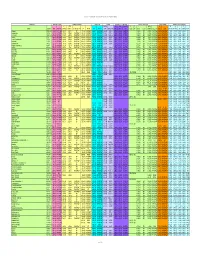
Chemical-Specific Parameters Supporting Table May 2016 Analyte
Regional Screening Level (RSL) Chemical-specific Parameters Supporting Table May 2016 Contaminant Molecular Weight Volatility Parameters Melting Point Density Diffusivity in Air and Water Partition Coefficients Water Solubility Tapwater Dermal Parameters H` (atm- Density Dia Diw Dia and Diw Kd Kd Koc log Kow S B τevent t* Kp 3 3 2 2 Analyte CAS No. MW MW Ref (unitless) m /mole) H` and HLC Ref VP VP Ref MP MP Ref (g/cm ) Density Ref (cm /s) (cm /s) Ref (L/kg) Ref (L/kg) Koc Ref (unitless) log Kow Ref (mg/L) S Ref (unitless) (hr/event) (hr) (cm/hr) K Ref Acephate 30560-19-1 1.8E+02 PHYSPRO 2.0E-11 5.0E-13 EPI 1.7E-06 PHYSPROP 8.8E+01 PHYSPROP 1.4E+00 CRC89 3.7E-02 8.0E-06 WATER9 1.0E+01 EPI -8.5E-01 PHYSPRO 8.2E+05 PHYSPROP 2.1E-04 1.1E+00 2.7E+00 4.0E-05 EPI Acetaldehyde 75-07-0 4.4E+01 PHYSPRO 2.7E-03 6.7E-05 PHYSPROP 9.0E+02 PHYSPROP -1.2E+02 PHYSPROP 7.8E-01 CRC89 1.3E-01 1.4E-05 WATER9 1.0E+00 EPI -3.4E-01 PHYSPRO 1.0E+06 PHYSPROP 1.3E-03 1.9E-01 4.5E-01 5.3E-04 EPI Acetochlor 34256-82-1 2.7E+02 PHYSPRO 9.1E-07 2.2E-08 PHYSPROP 2.8E-05 PHYSPROP 1.1E+01 PubChem 1.1E+00 PubChem 2.2E-02 5.6E-06 WATER9 3.0E+02 EPI 3.0E+00 PHYSPRO 2.2E+02 PHYSPROP 3.1E-02 3.4E+00 8.2E+00 5.0E-03 EPI Acetone 67-64-1 5.8E+01 PHYSPRO 1.4E-03 3.5E-05 PHYSPROP 2.3E+02 PHYSPROP -9.5E+01 PHYSPROP 7.8E-01 CRC89 1.1E-01 1.2E-05 WATER9 2.4E+00 EPI -2.4E-01 PHYSPRO 1.0E+06 PHYSPROP 1.5E-03 2.2E-01 5.3E-01 5.1E-04 EPI Acetone Cyanohydrin 75-86-5 8.5E+01 PHYSPRO 8.1E-08 2.0E-09 PHYSPROP 3.4E-01 PHYSPROP -1.9E+01 PHYSPROP 9.3E-01 CRC89 8.6E-02 1.0E-05 WATER9 1.0E+00 -

The Comparative Effects of Calcium Carbonate and of Calcium Silicate on the Yield of Sudan Grass Grown in a Ferruginous Latosol and a Hydrol Humic Latosol
TECHNICAL BULLETIN No. 53 JUNE 1963 The Comparative Effects of Calcium Carbonate and of Calcium Silicate on the Yield of Sudan Grass Grown in a Ferruginous Latosol and a Hydrol Humic Latosol N. H. MONTEITH and G. DONALD SHERMAN HAWAII AGRICULTURAL EXPERIMENT STATION, UNIVERSITY OF HAWAII The Comparative Effects of Calcium Carbonate and of Calcium Silicate on the Yield of Sudan Grass Grown in a Ferruginous Latosol and a Hydrol Humic Latosol N. H. MONTEITH and G. DONALD SHERMAN UNIVERSITY OF HAWAII COLLEGE OF TROPICAL AGRICULTURE HAWAII AGRICULTURAL EXPERIMENT STATION HONOLULU, H AWAII J UNE 1963 T ECIINICAL B ULLETIN No. 53 ACKNOWLEDGMENT The authors gra tcfully acknow ledge the assistance of the staff of th e Experiment Station of the H awaii an Sugar Planters' Association in pro viding greenhouse, photographic, and laboratory facilities, and for advice on sta tistical and analytical methods. Research funds on this proj ect were pro vid ed by the Hawaiian Sugar Planters' Association Experiment Station under a coope rative research agreemcnt with the Department of Agronomy and Soil Science. Funds and materials we re also provided by the Tenn essee Valley Authority, Contract No. TV21132A. THE AUTHORS N. H. MONTEITH was In structor in Agricultur e, University of Hawaii, 1961-1962. DR. G. DONALD SHERMAN, Associate Director of the Hawaii Agricultural Experiment Station, is Senior Soil Scientist at the Hawaii Agricultural Ex periment Station and Senior Professor of Soil Science, University of Hawaii. CONTENTS PAGE INTRODUcrION 5 LITERAT UHE REVIEW 5 Effect of Calcium Carbonate on Phosphorus Availability 5 Effect of Calcium Carbonate on Other Factors 7 Effect of Calcium Silicate on Phosphorus Availability 8 Effect of Calcium Silicate on Other Factors 8 EXPEmMENTAL PROCEDUHES 9 Soils . -

Environmental Health Criteria 206 Methyl Tertiary-Butyl Ether -J
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY Environmental Health Criteria 206 Methyl tertiary-Butyl Ether -J. l \r LNTER-ORGAN1ZATON PROGRAMME FOR THE SOUND MANAGEMENT OF CHEMICALS I JiVJ A cooperative agreement among UNEP, ILO, FAO, WHO, UNIDO, UNITAR and OECD THE ENVIRONMENTAL HEALTH CRITERIA SERIES Acetaldehyde (No. 167, 1995) Chiorofluorocarbons, partially halogenated Acetonitrile (No. 154, 1993) (ethane derivatives) (No. 139, 1992) Acrolein (No. 127, 1991) (methane derivatives) (No. 126, 1991) Acrylamide (No. 49, 1985) Chloroform (No. 163, 1994) Acrylic acid (No. 191, 1997) Chlorophenols (No. 93, 1989) Acrylonitrile (No. 28, 1983) Chlorothalonil (No. 183, 1996) Aged population, principles for evaluating Chromium (No. 61, 1988) the effects of chemicals (No. 144, 1992) Chrysotile asbestos (No. 203, 1998) Aldicarb (No. 121, 1991) Copper (No. 200, 1998) Aldrin and dieldrin (No. 91, 1989) Cresols (No. 168, 1995) Allethrins (No. 87, 1989) Cyhalothrin (No. 99, 1990) Aluminium (No. 194, 1997) Cypermethrin (No. 82, 1989) Amitrole (No. 158, 1994) Cypermethrin, alpha- (No. 142, 1992) Ammonia (No. 54, 1986) DOT and its derivatives (No. 9, 1979) Anticoagulant rodenticides (No. 175, 1995) DOT and its derivatives - Arsenic (No. 18, 1981) environmental aspects (No, 83, 1989) Asbestos and other natural mineral fibres Deltamethrjn (No. 97, 1990) (No. 53, 1986) Demeton-S-methyl (No. 197, 1997) Barium (No. 107, 1990) Diaminotoluenes (No. 74, 1987) Benomyl (No. 148, 1993) Diazinon (No. 198, 1997) Benzene (No. 150, 1993) 1,2-Dibromoethane (No. 177, 1996) Beryllium (No. 106, 1990) Oi-n-butyl phthalate (No. 189, 1997) Biomarkers and risk assessment: concepts 1 ,2-Dichloroethane and principles (No. 155, 1993) (No. 62, 1987, 1St edition) Blotoxins, aquatic (marine and freshwater) (No. -
![Ehealth DSI [Ehdsi V2.2.2-OR] Ehealth DSI – Master Value Set](https://docslib.b-cdn.net/cover/8870/ehealth-dsi-ehdsi-v2-2-2-or-ehealth-dsi-master-value-set-1028870.webp)
Ehealth DSI [Ehdsi V2.2.2-OR] Ehealth DSI – Master Value Set
MTC eHealth DSI [eHDSI v2.2.2-OR] eHealth DSI – Master Value Set Catalogue Responsible : eHDSI Solution Provider PublishDate : Wed Nov 08 16:16:10 CET 2017 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 1 of 490 MTC Table of Contents epSOSActiveIngredient 4 epSOSAdministrativeGender 148 epSOSAdverseEventType 149 epSOSAllergenNoDrugs 150 epSOSBloodGroup 155 epSOSBloodPressure 156 epSOSCodeNoMedication 157 epSOSCodeProb 158 epSOSConfidentiality 159 epSOSCountry 160 epSOSDisplayLabel 167 epSOSDocumentCode 170 epSOSDoseForm 171 epSOSHealthcareProfessionalRoles 184 epSOSIllnessesandDisorders 186 epSOSLanguage 448 epSOSMedicalDevices 458 epSOSNullFavor 461 epSOSPackage 462 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 2 of 490 MTC epSOSPersonalRelationship 464 epSOSPregnancyInformation 466 epSOSProcedures 467 epSOSReactionAllergy 470 epSOSResolutionOutcome 472 epSOSRoleClass 473 epSOSRouteofAdministration 474 epSOSSections 477 epSOSSeverity 478 epSOSSocialHistory 479 epSOSStatusCode 480 epSOSSubstitutionCode 481 epSOSTelecomAddress 482 epSOSTimingEvent 483 epSOSUnits 484 epSOSUnknownInformation 487 epSOSVaccine 488 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 3 of 490 MTC epSOSActiveIngredient epSOSActiveIngredient Value Set ID 1.3.6.1.4.1.12559.11.10.1.3.1.42.24 TRANSLATIONS Code System ID Code System Version Concept Code Description (FSN) 2.16.840.1.113883.6.73 2017-01 A ALIMENTARY TRACT AND METABOLISM 2.16.840.1.113883.6.73 2017-01 -

Ep 1112960 A1
Europäisches Patentamt *EP001112960A1* (19) European Patent Office Office européen des brevets (11) EP 1 112 960 A1 (12) EUROPEAN PATENT APPLICATION published in accordance with Art. 158(3) EPC (43) Date of publication: (51) Int Cl.7: C01F 7/00, C01B 33/38, 04.07.2001 Bulletin 2001/27 C07C 51/41, C07C 53/126, C01B 25/45, C09K 5/14, (21) Application number: 00944340.9 C08K 3/18, C08K 5/098, (22) Date of filing: 07.07.2000 B01J 41/08, B01J 41/10 (86) International application number: PCT/JP00/04554 (87) International publication number: WO 01/04053 (18.01.2001 Gazette 2001/03) (84) Designated Contracting States: • Igarashi, Hiroshi AT BE CH CY DE DK ES FI FR GB GR IE IT LI LU Mizusawa Industrial Chem., Ltd. MC NL PT SE Tokyo 103-0022 (JP) Designated Extension States: • Kondo, Masami AL LT LV MK RO SI Mizusawa Industrial Chemicals, Ltd. Tokyo 103-0022 (JP) (30) Priority: 08.07.1999 JP 19511799 • Minagawa, Madoka Mizusawa Industrial Chem., Ltd. (71) Applicant: Mizusawa Industrial Chemicals Ltd. Tokyo 103-0022 (JP) Tokyo 103-0022 (JP) • Sato, Tetsu Mizusawa Industrial Chemicals, Ltd. Tokyo 103-0022 (JP) (72) Inventors: • Sato, Teiji Mizusawa Industrial Chemicals, Ltd. • Komatsu, Yoshinobu Tokyo 103-0022 (JP) Mizusawa Industrial Chem. Ltd. Tokyo 103-0022 (JP) (74) Representative: Benson, John Everett • Ishida, Hitoshi J. A. Kemp & Co., Mizusawa Industrial Chemicals, Ltd 14 South Square, Tokyo 103-0022 (JP) Gray’s Inn London WC1R 5JJ (GB) (54) COMPOSITE POLYBASIC SALT, PROCESS FOR PRODUCING THE SAME, AND USE (57) A composite metal polybasic salt containing a trivalent metal and magnesium as metal components and having a novel crystal structure, and a method of preparing the same. -

3258 N:O 1179
3258 N:o 1179 LIITE 1 BILAGA 1 LÄÄKELUETTELON AINEET ÄMNENA I LÄKEMEDELSFÖRTECKNINGEN Latinankielinen nimi Suomenkielinen nimi Ruotsinkielinen nimi Englanninkielinen nimi Latinskt namn Finskt namn Svenskt namn Engelskt namn Abacavirum Abakaviiri Abakavir Abacavir Abciximabum Absiksimabi Absiximab Abciximab Acamprosatum Akamprosaatti Acamprosat Acamprosate Acarbosum Akarboosi Akarbos Acarbose Acebutololum Asebutololi Acebutolol Acebutolol Aceclofenacum Aseklofenaakki Aceklofenak Aceclofenac Acediasulfonum natricum Asediasulfoninatrium Acediasulfonnatrium Acediasulfone sodium Acepromazinum Asepromatsiini Acepromazin Acepromazine Acetarsolum Asetarsoli Acetarsol Acetarsol Acetazolamidum Asetatsoliamidi Acetazolamid Acetazolamide Acetohexamidum Asetoheksamidi Acetohexamid Acetohexamide Acetophenazinum Asetofenatsiini Acetofenazin Acetophenazine Acetphenolisatinum Asetofenoli-isatiini Acetfenolisatin Acetphenolisatin Acetylcholini chloridum Asetyylikoliinikloridi Acetylkolinklorid Acetylcholine chloride Acetylcholinum Asetyylikoliini Acetylkolin Acetylcholini Acetylcysteinum Asetyylikysteiini Acetylcystein Acetylcysteine Acetyldigitoxinum Asetyylidigitoksiini Acetyldigitoxin Acetyldigitoxin Acetyldigoxinum Asetyylidigoksiini Acetyldigoxin Acetyldigoxin Acetylisovaleryltylosini Asetyyli-isovaleryyli- Acetylisovaleryl- Acetylisovaleryltylosine tartras tylosiinitartraatti tylosintartrat tartrate Aciclovirum Asikloviiri Aciklovir Aciclovir Acidum acetylsalicylicum Asetyylisalisyylihappo Acetylsalicylsyra Acetylsalicylic acid Acidum alendronicum -

TOXICOLOGICAL PROFILE for METHYL Tert-BUTYL ETHER
TOXICOLOGICAL PROFILE FOR METHYL tert-BUTYL ETHER U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES Public Health Service Agency for Toxic Substances and Disease Registry August 1996 METHYL tert-BUTYL ETHER ii DISCLAIMER The use of company or product name(s) is for identification only and does not imply endorsement by the Agency for Toxic Substances and Disease Registry. METHYL tert-BUTYL ETHER iii UPDATE STATEMENT Toxicological profiles are revised and republished as necessary, but no less than once every three years. For information regarding the update status of previously released profiles, contact ATSDR at: Agency for Toxic Substances and Disease Registry Division of Toxicology/Toxicology Information Branch 1600 Clifton Road NE, E-29 Atlanta, Georgia 30333 vi *Legislative Background The toxicological profiles are developed in response to the Superfund Amendments and Reauthorization Act (SARA) of 1986 (Public Law 99-499) which amended the Comprehensive Environmental Response, Compensation, and Liability Act of 1980 (CERCLA or Superfund). This public law directed ATSDR to prepare toxicological profiles for hazardous substances most commonly found at facilities on the CERCLA National Priorities List and that pose the most significant potential threat to human health, as determined by ATSDR and the EPA. The availability of the revised priority list of 275 hazardous substances was announced in the Federal Register on April 29, 1996 (61 FR 18744). For prior versions of the list of substances, see Federal Register notices dated April 17, 1987 (52 FR 12866); October 20, 1988 (53 FR 41280); October 26, 1989 (54 FR 43619); October 17, 1990 (55 FR 42067); October 17, 199l (56 FR 52166); October 28, 1992 (57 FR 48801); and February 28, 1994 (59 FR 9486).