Coastal Fish Assemblages and Predation Pressure in Northern
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

FIP N° 2003-22 CARACTERIZACIÓN ECOLÓGICA Y PESQUERA DEL ÁREA DE RESERVA ARTESANAL ENTRE LA I Y II REGIONES
INFORME FINAL FIP N° 2003-22 Caracterización ecológica y pesquera del área de reserva artesanal entre la I y II Regiones • Junio, 2005 • REQUIRENTE FONDO DE INVESTIGACIÓN PESQUERA, FIP Presidente del Consejo: Felipe Sandoval Precht EJECUTOR INSTITUTO DE FOMENTO PESQUERO, IFOP Jefe División Investigación Pesquera: Mauricio Braun Alegría Director Ejecutivo: Guillermo Moreno Paredes • Junio, 2005 • JEFE DE PROYECTO JORGE GONZÁLEZ YÁÑEZ. AUTORES JORGE GONZÁLEZ Y. MÓNICA CATRILAO C. NANCY BARAHONA T. CARLOS MARTÍNEZ F. CARLOS TAPIA J. ÁLVARO WILSON M. JORGE GARRIDO P. VÍCTOR BAROS P. ZAIDA YOUNG U. CARLOS CORTES S. CÉSAR GUEVARA P. CARLOS GASPAR S. JUAN SAAVEDRA N. • Junio, 2005 • INSTITUTO DE FOMENTO PESQUERO / DIVISIÓN DE INVESTIGACIÓN PESQUERA RESUMEN EJECUTIVO El sector pesquero de la I y II Regiones, comprende un importante segmento de la economía nacional, contribuyendo fuertemente con el empleo en las zonas costeras y al abastecimiento de materia prima. En esta macrozona la pesca está representada por dos sectores: industrial y artesanal y a su vez está constituida por tres segmentos principales de recursos; pesquerías bentónicas, pesquerías de peces y recolección de algas. Dada la importancia de la actividad extractiva artesanal e industrial en la franja de las 5 millas de la I y II Regiones, en el presente informe se describe y analiza la actividad extractiva de las principales pesquerías. Además, se entrega una completa revisión de los principales antecedentes eco-pesqueros del litoral de las regiones I y II, los cuales en conjunto con la información generada en el marco del proyecto, permitió generar una base de información georreferenciada, actualizada e integrada, para la franja de las 5 millas. -

Diets and Coexistence of the Sea Urchins Lytechinus Variegatus and Arbacia Punctulata (Echinodermata) Along the Central Florida Gulf Coast
MARINE ECOLOGY PROGRESS SERIES Vol. 295: 171–182, 2005 Published June 23 Mar Ecol Prog Ser Diets and coexistence of the sea urchins Lytechinus variegatus and Arbacia punctulata (Echinodermata) along the central Florida gulf coast Janessa Cobb, John M. Lawrence* Department of Biology, University of South Florida, Tampa, Florida 33620, USA ABSTRACT: The basis for coexistence of similar species is fundamental in community ecology. One mechanism for coexistence is differentiation of diets. Lytechinus variegatus and Arbacia punctulata coexist in different microhabitats along the Florida gulf coast. Their great difference in morphology might affect their choice of microhabitats and diet. We analyzed diets of both species at 1 offshore and 1 nearshore site where both occurred in relatively equal numbers, an offshore site dominated by A. punctulata and an offshore site dominated by L. variegatus. Gut contents were analyzed to deter- mine the diet. A. punctulata prim. consumed sessile invertebrates except on dates when algal avail- ability was higher than normal. L. variegatus primarily consumed macroflora except on dates when macroflora was extremely limited. Electivity indices revealed no strong preferences for particular species of algae, although L. variegatus consumed many drift species. A. punctulata and L. variega- tus both fed in a random manner, although they avoided particular species of algae known to contain high concentrations of secondary metabolites. The diet of A. punctulata was correlated with algae only over rubble outcroppings at the offshore site with the highest biomass. Diets of offshore popula- tions were more similar to each other, regardless of the presence of conspecifics, than to those of populations at Caspersen Beach (nearshore site). -

Universidad Austral De Chile Facultad De Ciencias Escuela De Biología Marina
Universidad Austral de Chile Facultad de Ciencias Escuela de Biología Marina Profesor Patrocinante: Dr. Dirk Schories. Instituto de Ciencias Marinas y Limnológicas. Facultad de Ciencias – Universidad Austral de Chile. Profesor Co-patrocinante: Dr. Luis M. Pardo. Instituto de Ciencias Marinas y Limnológicas. Facultad de Ciencias – Universidad Austral de Chile. ECOLOGÍA TRÓFICA DEL ASTEROIDEO Cosmasterias lurida (Phillipi, 1858) EN EL SENO DEL RELONCAVÍ (SUR DE CHILE): DISTRIBUCIÓN, ABUNDANCIA, ALIMENTACIÓN Y MOVIMIENTO. Tesis de Grado presentada como parte de los requisitos para optar al grado de Licenciado en Biología Marina y Título Profesional de Biólogo Marino. IGNACIO ANDRÉS GARRIDO IRIONDO VALDIVIA - CHILE 2012. AGRADECIMIENTOS Primero que todo, me siento extremadamente afortunado gracias a tanta gente maravillosa que en estos 25 años se ha cruzado por mi camino. Quiero agradecer especialmente a todos los que aportaron de alguna forma en mi formación como Biólogo Marino: A mi núcleo familiar, Margarita I., Dagoberto G. y Augusto G. (también Gorlak y Ulises) que con sus consejos y apoyo incondicional logre cumplir este sueño que tanto anhelaba. Gracias por todo el cariño y por creer en mí, esto se los dedico a ustedes. Al Dr. Dirk Schories, amigo y profesor, quien me enseño a disfrutar y valorar lo que más admiro en la vida, la naturaleza y el infinito mundo submarino. Asimismo, quien me guió en mi formación como Biólogo Marino y con quien compartí incontables inmersiones fascinantes e inolvidables. Además fue quien financio esta tesis de pregrado. Espero podamos continuar trabajando en el futuro. ¡Muchas gracias por todo! Al Dr. Luis M. Pardo, quien con el tiempo se convirtió en un importante guía profesional y amigo. -

Fecha: 16 / 05 / 2018 /
Ficha de Claves Gráficas para el Reconocimiento de Peces de Roca Número de ficha: Ficha 02 Fecha: 16 / 05 / 2018 / 1. Especie/ Estado de desarrollo Nombre Común Nombre Científico Rollizo Pinguipes Chilensis (Valenciennes, 1833) Reino Animalia (Kingdom) Clase Actinopterygii (Class) Filo Chordata (Phylum) Orden Perciformes (Order) Subfilo Vertebrata (Subphylum) Suborden Trachinoidei (Suborder) Superclase Gnathostomata (Superclass) Familia Pinguipedidae (Family) Superclase Pisces (Superclass) Género Pinguipes (Genus) Especie Pinguipes chilensis (Species) Fuente: Froese, R. and D. Pauly. Editors. (2018). FishBase. Pinguipes chilensis Valenciennes, 1833. Accessed through: World Register of Marine Species at: http://marinespecies.org/aphia.php?p=taxdetails&id=279407 Fuente: https://www.fishbase.de/summary/12942 2. Distribución Geográfica/Profundidad en la que habita/ Característica del Hábitat Desde Arica hasta Puerto Montt, y Chiloé. En todos lados son iguales, no cambia por lugar. En el sur los encuentras a medio metro. Desde ½ metro hasta 30 metros. (Yo no sé más allá de los 30 metros. Pero no creo que estén más abajo, si abajo no hay comida, la comida está arriba. Las algas llegan a los 25 metros, más abajo no hay. Están en los lugares planos, pero también en los huiros y también se encuevan. 3. Rango de Luminosidad del medio/ En la figura 2, están con flash, aunque estén a 7 metros. 4. Rasgo Sobresaliente sobre el Color del pez respecto al Medio 1 Los puntitos reflejan la luz. La boca amarilla, entre más viejos más amarilla la boca. Se ponen como patos. Es medio gris, como un gris más claro, pero no es morado. Tampoco es plateado. Las castañetas son plateadas. -

Final FIP 2003-22.Pdf
Caracterización ecológica y pesquera del área de reserva artesanal entre la I y II Regiones Item Type Report Authors González, J.; Catrilao, M.; Barahona, N.; Martínez, C.; Tapia, C.; Wilson, A.; Garrido, J.; Baros, V.; Young, Z.; Cortes, C.; Guevara, C.; Gaspar, C.; Saavedra, J. Download date 10/10/2021 21:10:40 Link to Item http://hdl.handle.net/1834/1811 INFORME FINAL FIP N° 2003-22 Caracterización ecológica y pesquera del área de reserva artesanal entre la I y II Regiones • Junio, 2005 • REQUIRENTE FONDO DE INVESTIGACIÓN PESQUERA, FIP Presidente del Consejo: Felipe Sandoval Precht EJECUTOR INSTITUTO DE FOMENTO PESQUERO, IFOP Jefe División Investigación Pesquera: Mauricio Braun Alegría Director Ejecutivo: Guillermo Moreno Paredes • Junio, 2005 • JEFE DE PROYECTO JORGE GONZÁLEZ YÁÑEZ. AUTORES JORGE GONZÁLEZ Y. MÓNICA CATRILAO C. NANCY BARAHONA T. CARLOS MARTÍNEZ F. CARLOS TAPIA J. ÁLVARO WILSON M. JORGE GARRIDO P. VÍCTOR BAROS P. ZAIDA YOUNG U. CARLOS CORTES S. CÉSAR GUEVARA P. CARLOS GASPAR S. JUAN SAAVEDRA N. • Junio, 2005 • INSTITUTO DE FOMENTO PESQUERO / DIVISIÓN DE INVESTIGACIÓN PESQUERA RESUMEN EJECUTIVO El sector pesquero de la I y II Regiones, comprende un importante segmento de la economía nacional, contribuyendo fuertemente con el empleo en las zonas costeras y al abastecimiento de materia prima. En esta macrozona la pesca está representada por dos sectores: industrial y artesanal y a su vez está constituida por tres segmentos principales de recursos; pesquerías bentónicas, pesquerías de peces y recolección de algas. Dada la importancia de la actividad extractiva artesanal e industrial en la franja de las 5 millas de la I y II Regiones, en el presente informe se describe y analiza la actividad extractiva de las principales pesquerías. -

Guía De Peces Litorales
PECES LITORALES de Chile Guía para una pesca recreativa marina sustentable Conservar las GUÍA PARA UNA PESCA RECREATIVA MARINA SUSTENTABLE tierras y aguas de las cuales depende la vida. Publicación realizada por The Nature Conservancy Este trabajo es producto de la colaboración de expertos académicos, de instituciones del estado, federaciones de pesca recreativa y ONGs. Santiago de Chile, 2020 Nature.org/LatinAmerica nature_org nature_org thenatureconservancy - 3 - GUÍA PARA UNA PESCA RECREATIVA MARINA SUSTENTABLE La pesca recreativa una actividad sustentable The Nature Conservancy (TNC) es una Organización No de Chile. Este grupo de especies incluyen especies carnívoras Gubernamental (ONG) con la misión de conservar y proteger y micro-carnívoras tales como la Vieja Negra o Mulato (Graus las aguas y tierras de las que depende la vida en las princi- nigra), Pejeperro (Semicossyphus darwini), Apañao (Hemilut- pales regiones del planeta, en línea con el desarrollo social, janus macrophthalmos) y Bilagay o Pintacha (Cheilodactylus económico y el respeto por las comunidades. En Chile y Perú, variegatus); omnívoros como el Acha (Medialuna ancietae) y el estamos trabajando en proteger y dar un uso sustentable a Baunco (Girella laevifrons), y el herbívoro Jerguilla (Aplodactylus los recursos pesqueros que son parte del ecosistema mari- punctatus), entre otros. Las especies más emblemáticas del no de la Corriente de Humboldt, especialmente de los peces litoral chileno son tres; la Vieja Negra o Mulato, el Pejeperro litorales y del hábitat marino costero que generan las algas y el Acha y son las que muestran los mayores problemas de pardas. conservación, debido a que alcanzan grandes tamaños y va- lor comercial. -

Redalyc.Revisión Bibliográfica De Especies Ectoparásitas Y
Revista de Biología Marina y Oceanografía ISSN: 0717-3326 [email protected] Universidad de Valparaíso Chile Muñoz, Gabriela; Olmos, Viviana Revisión bibliográfica de especies ectoparásitas y hospedadoras de sistemas acuáticos de Chile Revista de Biología Marina y Oceanografía, vol. 42, núm. 2, agosto, 2007, pp. 89-148 Universidad de Valparaíso Viña del Mar, Chile Disponible en: http://www.redalyc.org/articulo.oa?id=47942201 Cómo citar el artículo Número completo Sistema de Información Científica Más información del artículo Red de Revistas Científicas de América Latina, el Caribe, España y Portugal Página de la revista en redalyc.org Proyecto académico sin fines de lucro, desarrollado bajo la iniciativa de acceso abierto Revista de Biología Marina y Oceanografía 42(2): 89 – 148, agosto de 2007 Revisión bibliográfica de especies ectoparásitas y hospedadoras de sistemas acuáticos de Chile Bibliographic revision of ectoparasite and host species from aquatic systems of Chile Gabriela Muñoz1 y Viviana Olmos2 1Facultad de Ciencias del Mar y de Recursos Naturales, Universidad de Valparaíso, Casilla 5080, Reñaca, Viña del Mar, Chile 2Departamento de Zoología, Facultad de Ciencias, Universidad de Concepción, Casilla 160-C, Concepción, Chile [email protected] Abstract.- To date there are about 380 scientific Resumen.- Actualmente existen cerca de 380 publications on eumetazoan parasites collected from aquatic publicaciones científicas referidas a parásitos eumetazoos animals from Chile. However, all this information is spread obtenidos de animales acuáticos recolectados a lo largo de on different bibliographic sources including some old articles Chile. Sin embargo, esta literatura está dispersa en distintas (a century old). Few studies have summarized in checklists fuentes bibliográficas; algunas con más de un siglo de the surveys of some groups of parasite and host species. -

GAYANA Genetic Diversity and Demographic History of the Endemic Southeastern Pacific Sea Urchin Arbacia Spatuligera
GAYANA Gayana (2019) vol. 83, No. 2, 81-92 DOI: 10.4067/S0717-65382019000200081 ORIGINAL ARTICLE Genetic diversity and demographic history of the endemic Southeastern Pacific sea urchin Arbacia spatuligera (Valenciennes 1846) Diversidad genética e historia demográfica del erizo de mar endémico del Pacífico Sureste Arbacia spatuligera (Valenciennes 1846) Constanza Millán1, Angie Díaz1,2,*, Elie Poulin2,3, Catalina Merino-Yunnissi4 & Andrea Martínez4 1Laboratorio de Ecología Molecular Marina (LEMMAR), Departamento de Zoología, Facultad de Ciencias Naturales y Oceanográficas, Universidad de Concepción, Concepción, Chile. 2Instituto de Ecología y Biodiversidad (IEB), Departamento de Ciencias Ecológicas, Universidad de Chile, Santiago, Chile. 3Laboratorio de Ecología Molecular, Departamento de Ciencias Ecológicas, Facultad de Ciencias, Universidad de Chile, Santiago, Chile. 4Departamento de Zoología de Invertebrados, Museo Nacional de Historia Natural, Santiago, Chile. *Email: [email protected] ABSTRACT The pattern of the genetic structuring of marine species result from the relationship between homogenizing and structuring factors, together with historical and contemporary processes. Dispersal potential has been described as a homogenizing factor, corroborated by the connectivity paradigm, which states that high dispersers show low or no genetic differentiation. In contrast, biogeographic breaks and oceanic currents have an important role in limiting or enhancing connectivity, being structuring factors. We studied this relationship in Arbacia spatuligera, a subtidal echinoid with a planktonic larval stage, which is distributed along the Southeastern Pacific (SEP). The SEP is divided into two biogeographic provinces with an Intermediate Area between both them, which is delimited by two biogeographic breaks (~30° S and 40°-42° S). Moreover, much of the SEP coast, from ~42° S to 6° S, it is influenced by a complex system of marine currents known as the Humboldt Current System (HCS). -

The Diet and Predator-Prey Relationships of the Sea Star Pycnopodia Helianthoides (Brandt) from a Central California Kelp Forest
THE DIET AND PREDATOR-PREY RELATIONSHIPS OF THE SEA STAR PYCNOPODIA HELIANTHOIDES (BRANDT) FROM A CENTRAL CALIFORNIA KELP FOREST A Thesis Presented to The Faculty of Moss Landing Marine Laboratories San Jose State University In Partial Fulfillment of the Requirements for the Degree Master of Arts by Timothy John Herrlinger December 1983 TABLE OF CONTENTS Acknowledgments iv Abstract vi List of Tables viii List of Figures ix INTRODUCTION 1 MATERIALS AND METHODS Site Description 4 Diet 5 Prey Densities and Defensive Responses 8 Prey-Size Selection 9 Prey Handling Times 9 Prey Adhesion 9 Tethering of Calliostoma ligatum 10 Microhabitat Distribution of Prey 12 OBSERVATIONS AND RESULTS Diet 14 Prey Densities 16 Prey Defensive Responses 17 Prey-Size Selection 18 Prey Handling Times 18 Prey Adhesion 19 Tethering of Calliostoma ligatum 19 Microhabitat Distribution of Prey 20 DISCUSSION Diet 21 Prey Densities 24 Prey Defensive Responses 25 Prey-Size Selection 27 Prey Handling Times 27 Prey Adhesion 28 Tethering of Calliostoma ligatum and Prey Refugia 29 Microhabitat Distribution of Prey 32 Chemoreception vs. a Chemotactile Response 36 Foraging Strategy 38 LITERATURE CITED 41 TABLES 48 FIGURES 56 iii ACKNOWLEDGMENTS My span at Moss Landing Marine Laboratories has been a wonderful experience. So many people have contributed in one way or another to the outcome. My diving buddies perse- vered through a lot and I cherish our camaraderie: Todd Anderson, Joel Thompson, Allan Fukuyama, Val Breda, John Heine, Mike Denega, Bruce Welden, Becky Herrlinger, Al Solonsky, Ellen Faurot, Gilbert Van Dykhuizen, Ralph Larson, Guy Hoelzer, Mickey Singer, and Jerry Kashiwada. Kevin Lohman and Richard Reaves spent many hours repairing com puter programs for me. -

Actualización Y Validación De La Clasificación De Las Zonas Biogeográficas Litorales
Proyecto FIP N° 2004 – 28 INFORME FINAL ACTUALIZACIÓN Y VALIDACIÓN DE LA CLASIFICACIÓN DE LAS ZONAS BIOGEOGRÁFICAS LITORALES UNIVERSIDAD AUSTRAL DE CHILE Enero de 2006 RESUMEN EJECUTIVO El objetivo general de este Proyecto fue actualizar la clasificación de las zonas zoogeográficas litorales de la costa de Chile, determinando los factores físicos y biológicos que permiten caracterizar esas zonas. Se analizaron bases de datos de las características físicas de la costa de Chile, incluyendo rugosidad de la costa, ancho de la plataforma continental, vientos costeros, temperatura superficial del mar, radiación solar y concentración (satelital) de clorofila y de la distribución y abundancia de invertebrados y peces de ambientes inter y submareales y de fondos blandos y rocosos y base de datos de macroalgas. a) Características físicas y ambientales de la costa de Chile La costa de Chile puede ser dividida en tres grandes zonas de acuerdo a la rugosidad de la misma: (i) una costa extremadamente rugosa entre 51 y 55ºS, (ii) una costa rugosa entre 46 y 50ºS y (iii) una costa poco rugosa desde 40ºS hacia el norte. Cuatro zonas se pueden reconocer en base al ancho de la plataforma: (i) plataforma muy angosta (< 10 km) entre el límite norte y 33,5ºS; (ii) plataforma menos angosta (>10 km, < 20 km) entre 33,5ºS y 36ºS; (iii) plataforma ancha (hasta 70 km) entre 36ºS hasta 40ºS; (iv) ancho dificilmente definible al sur de 40ºS, por la extrema rugosidad de la zona. Desde el punto de vista de los vientos, se tienen al menos 3 zonas geográficas: (i) la zona centro- norte del país, desde el límite norte del país hasta 39ºS, con vientos meridionales hacia el norte durante todo el año; (ii) una zona de transición entre 39 y 45ºS, en que la dirección de los vientos meridionales depende de la estación del año; e (iii) la zona austral del país, al sur de 45ºS, en que los vientos meridionales soplan hacia el sur durante todo el año. -
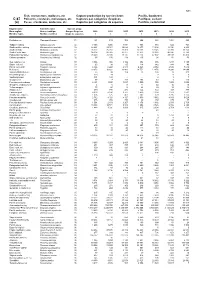
Fish, Crustaceans, Molluscs, Etc Capture Production by Species
531 Fish, crustaceans, molluscs, etc Capture production by species items Pacific, Southeast C-87 Poissons, crustacés, mollusques, etc Captures par catégories d'espèces Pacifique, sud-est (a) Peces, crustáceos, moluscos, etc Capturas por categorías de especies Pacífico, sudoriental English name Scientific name Species group Nom anglais Nom scientifique Groupe d'espèces 2009 2010 2011 2012 2013 2014 2015 Nombre inglés Nombre científico Grupo de especies t t t t t t t Flatfishes nei Pleuronectiformes 31 481 613 302 806 323 1 804 483 Tadpole codling Salilota australis 32 3 356 1 400 1 091 768 374 522 703 Southern blue whiting Micromesistius australis 32 22 221 23 301 19 629 16 675 15 304 10 036 8 809 Southern hake Merluccius australis 32 26 272 25 361 20 909 20 288 19 346 12 393 16 150 South Pacific hake Merluccius gayi 32 94 306 90 305 82 977 72 872 92 031 96 196 77 283 Patagonian grenadier Macruronus magellanicus 32 78 440 74 330 70 137 62 175 47 602 39 138 37 475 Chilean grenadier Coelorinchus chilensis 32 65 156 134 136 91 54 59 Sea catfishes nei Ariidae 33 3 096 406 1 426 852 876 1 217 1 185 Snake eels nei Ophichthidae 33 60 38 65 114 142 144 49 Pacific cornetfish Fistularia corneta 33 6 396 6 443 4 513 4 323 4 854 2 534 7 630 Mullets nei Mugilidae 33 18 673 10 821 13 400 18 751 13 876 14 290 14 044 Snooks(=Robalos) nei Centropomus spp 33 25 98 104 78 136 79 310 Broomtail grouper Mycteroperca xenarcha 33 120 14 .. -

Historia Física Y Política De Chile
21 Historia física Claudio Gay y política de Chile (1800-1873) La Biblioteca fundamentos de la construcción de Chile En los inicios de la república, cuando todo estaba El naturalista francés arribó a Chile en es una iniciativa de la Cámara Chilena de por hacerse, cuando Chile sólo existía como proyecto Zoología II 1828 para trabajar como profesor. En 1829, la Construcción, en conjunto con la Pon- institucional, ¿cómo era el territorio bajo la jurisdicción inició su labor de reconocimiento del terri- tificia Universidad Católica de Chile y la del nuevo Estado?, ¿cuáles, las características físicas, torio nacional. Un año después comenzó su Claudio Gay Dirección de Bibliotecas, Archivos y Museos económicas, culturales y sociales del conjunto bajo su trabajo más importante al suscribir un con- que, en formato impreso y multimedia, soberanía?, ¿cuál, la noción existente acerca del número Claudio Gay trato con el gobierno. En éste, el sabio se reúne las obras de los científicos, profesio- y distribución espacial de sus habitantes?, ¿cuáles, sus comprometió a hacer un viaje científico por nales y técnicos que con sus trabajos dieron principales recursos económicos?, ¿cuáles, sus carac- todo el territorio con el objeto de investigar a conocer Chile –sus recursos humanos y terísticas ambientales?, ¿sus potencialidades? A éstas, la historia natural de Chile y todo aquello naturales, así como sus características sociales y muchas otras interrogantes buscaba dar respuestas el que contribuyera a dar a conocer sus produc- y evolución histórica– contribuyendo con gobierno chileno cuando en 1830 decidió la contrata- tos. sus investigaciones, informes y trabajos a la ción de Claudio Gay.