Presence of Riparian Vegetation Increases Biotic Condition of Fish Assemblages in Two Brazilian Reservoirs
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Estrutura E Composição Da Ictiofauna De Riachos Do Rio Paranapanema, Sudeste E Sul Do Brasil
ESTRUTURA E COMPOSIÇÃO DA ICTIOFAUNA DE RIACHOS DO RIO PARANAPANEMA, SUDESTE E SUL DO BRASIL Ricardo M. C. Castro1; Lilian Casatti2; Hertz F. Santos1; Katiane M. Ferreira1; Alexandre C. Ribeiro1; Ricardo C. Benine1; Gabriela Z. P. Dardis1; Alex L. A. Melo1; Renata Stopiglia1; Tatiana X. Abreu1; Flávio A. Bockmann1; Murilo Carvalho1; Fernando Z. Gibran1 & Flávio C. T. Lima3 Biota Neotropica v3 (n1) –http://www.biotaneotropica.org.br/v3n1/pt/abstract?article+BN01703012003 Recebido em: 18/02/2003 Publicado em: 11/04/2003 1Laboratório de Ictiologia de Ribeirão Preto (LIRP), Departamento de Biologia da FFCLRP-USP (www.ffclrp.usp.br), Av. Bandeirantes 3900, 14040-901, Ribeirão Preto, SP, Brasil 2Departamento de Zoologia e Botânica, IBILCE, Universidade Estadual Paulista (www.ibilce.unesp.br), R. Cristóvão Colombo 2265, 15054-000, São José do Rio Preto, SP, Brasil 3Museu de Zoologia da Universidade de São Paulo, MZUSP (www.mz.usp.br), Caixa Postal 42594, 04299-970, São Paulo, SP, Brasil Autor para correspondência: Ricardo M. C. Castro (e-mail: [email protected]) Abstract Seventeen 100 m long streams stretches, none of an order higher than three, were sampled to both sides of the main channel of Rio Paranapanema in the States of São Paulo and Paraná, southeastern and southern Brazil. Each stream stretch had its midpoint located with a GPS satellite receiver and had its fish fauna sampled via a standardized environmental data and fish collection methodology (primarily utilizing electrofishing) with the aim of providing the following information about each stream: 1) the taxonomic composition of the fish fauna and the contribution of each species in that stream in terms of both number of individuals and biomass; 2) a photographic documentation of the live coloration of representative speci- mens of each collected species; and 3) the description of each sampled environment, with colored photographic illustra- tions and details of the main biotic and abiotic parameters. -

Ata Da Reunião Extraordinária Do Segmento Dos Municípios Do
Ata da Reunião Extraordinária do Segmento dos Municípios do Comitê da Bacia Hidrográfica do Tietê-Batalha no ano de 2021 referente ao processo de eleição para o biênio março de 2021 a março de 2023, de 25 de janeiro de 2021, resumo publicado no Diário Oficial do Estado em 16/02/2021. Aos vinte e cinco dias do mês de janeiro do ano de dois mil e vinte e um, às 10h, através da plataforma de videoconferência, Microsoft Teams, compareceram os membros do Comitê da Bacia Hidrográfica do Tietê Batalha para a realização da Reunião Extraordinária de Eleição do CBH/TB, segmento Municípios. Estiveram presentes 19 prefeitos (as) representando o segmento, com 9 titulares à saber: Vladimir Antonio Adabo (Prefeito Municipal de Borborema), Bruno Floriano de Oliveira (Prefeito Municipal de Guaiçara), Marcos Roberto Frugeri (Prefeito Municipal de Guarantã), Cesar Henrique da Cunha Fiala (Prefeito Municipal de Pirajuí), Gilhiard Herrique de Bortoli (Prefeito Municipal de Pongaí), Ronaldo Correa da Silva (Prefeito Municipal de Reginópolis), Eder Ruiz Magalhães de Andrade (Prefeito Municipal de Sabino), Robson Eduardo Fortes (Prefeito Municipal de Uru), Alcemir Cássio Gréggio (Prefeito Municipal de Urupês) e 10 suplentes à saber: Fernando Octaviani (Prefeito Municipal de Agudos), Hellen Fernandes Rodrigues Coelho (Prefeita Municipal de Avaí), Taís Fernanda M. Contieri Santana (Prefeita Municipal de Cafelândia), Cássio Roberto Bertelli (Prefeito Municipal de Elisiário), Reni Aparecida da Silva (Prefeita Municipal de Irapuã), Marcio Perpetuo Augusto (Prefeito Municipal de Marapoama), Hericson de Carvalho Lino (Prefeito Municipal de Mendonça), Cristiano dos Santos (Prefeito Municipal de Presidente Alves), Artur Manoel Nogueira Franco (Prefeito Municipal de Promissão) e Josemar Francisco de Abreu (Prefeito Municipal de Sales). -

MUNICÍPIO MÓDULO FISCAL - MF (Ha) (Ha)
QUATRO MFs MUNICÍPIO MÓDULO FISCAL - MF (ha) (ha) ADAMANTINA 20 80 ADOLFO 22 88 AGUAÍ 18 72 ÁGUAS DA PRATA 22 88 ÁGUAS DE LINDÓIA 16 64 ÁGUAS DE SANTA BÁRBARA 30 120 ÁGUAS DE SÃO PEDRO 18 72 AGUDOS 12 48 ALAMBARI 22 88 ALFREDO MARCONDES 22 88 ALTAIR 28 112 ALTINÓPOLIS 22 88 ALTO ALEGRE 30 120 ALUMÍNIO 12 48 ÁLVARES FLORENCE 28 112 ÁLVARES MACHADO 22 88 ÁLVARO DE CARVALHO 14 56 ALVINLÂNDIA 14 56 AMERICANA 12 48 AMÉRICO BRASILIENSE 12 48 AMÉRICO DE CAMPOS 30 120 AMPARO 20 80 ANALÂNDIA 18 72 ANDRADINA 30 120 ANGATUBA 22 88 ANHEMBI 30 120 ANHUMAS 24 96 APARECIDA 24 96 APARECIDA D'OESTE 30 120 APIAÍ 16 64 ARAÇARIGUAMA 12 48 ARAÇATUBA 30 120 ARAÇOIABA DA SERRA 12 48 ARAMINA 20 80 ARANDU 22 88 ARAPEÍ 24 96 ARARAQUARA 12 48 ARARAS 10 40 ARCO-ÍRIS 20 80 AREALVA 14 56 AREIAS 35 140 AREIÓPOLIS 16 64 ARIRANHA 16 64 ARTUR NOGUEIRA 10 40 ARUJÁ 5 20 ASPÁSIA 26 104 ASSIS 20 80 ATIBAIA 16 64 AURIFLAMA 35 140 AVAÍ 14 56 AVANHANDAVA 30 120 AVARÉ 30 120 BADY BASSITT 16 64 BALBINOS 20 80 BÁLSAMO 20 80 BANANAL 24 96 BARÃO DE ANTONINA 20 80 BARBOSA 30 120 BARIRI 16 64 BARRA BONITA 14 56 BARRA DO CHAPÉU 16 64 BARRA DO TURVO 16 64 BARRETOS 22 88 BARRINHA 12 48 BARUERI 7 28 BASTOS 16 64 BATATAIS 22 88 BAURU 12 48 BEBEDOURO 14 56 BENTO DE ABREU 30 120 BERNARDINO DE CAMPOS 20 80 BERTIOGA 10 40 BILAC 30 120 BIRIGUI 30 120 BIRITIBA-MIRIM 5 20 BOA ESPERANÇA DO SUL 12 48 BOCAINA 16 64 BOFETE 20 80 BOITUVA 18 72 BOM JESUS DOS PERDÕES 16 64 BOM SUCESSO DE ITARARÉ 20 80 BORÁ 20 80 BORACÉIA 16 64 BORBOREMA 16 64 BOREBI 12 48 BOTUCATU 20 80 BRAGANÇA PAULISTA 16 64 BRAÚNA -

Influence of Land Use Changes on Water Chemistry in Streams in the State of São Paulo, Southeast Brazil
Anais da Academia Brasileira de Ciências (2012) 84(4): 919-930 (Annals of the Brazilian Academy of Sciences) Printed version ISSN 0001-3765 / Online version ISSN 1678-2690 www.scielo.br/aabc Influence of land use changes on water chemistry in streams in the State of São Paulo, southeast Brazil DANIELA M.L. SILVA1, PLÍNIO B. CAMARGO2, WILLIAM H. MCDOWELL3, IVAN VIEIRA2, MARCOS S.M.B. SALOMÃO4 and LUIZ A. MARTINELLI2 1 Universidade Estadual de Santa Cruz, Departamento de Ciências Biológicas, Rodovia Ilhéus – Itabuna, Km 16, 45662-900 Ilhéus, BA, Brasil 2 Centro de Energia Nuclear na Agricultura, Universidade de São Paulo, Av. Centenário, 303, Caixa Postal 96, 13416-000 Piracicaba, SP, Brasil 3 Department of Natural Resources, University of New Hampshire, 05 Main Street, Durham, NH 03824, USA 4 Universidade Estadual do Norte Fluminense Darcy Ribeiro, Centro de Biociências e Biotecnologia, Av. Alberto Lamego, 2000, 28013-600 Campos dos Goytacazes, RJ, Brasil Manuscript received on May 31, 2011; accepted for publication on April 24, 2012 ABSTRACT Streamwater is affected by several processes in the watershed including anthropogenic activities that result in changes in water quality as well as in the functioning of these stream ecosystems. Therefore, this work aims to 2+ 2+ + + + - - - 2- 3- - evaluate the concentration of major ions (Ca , Mg , Na , K , NH4 , NO3 , NO2 , Cl , SO4 , PO4 , HCO3 ) in streams in the state of São Paulo (southeast Brazil). The sampling sites are located at undisturbed (ombrophilus dense forest, semideciduous forest and savanna – cerrado) and disturbed areas (pasture, urbanization and sugar cane crops). Streamwater chemistry varied according to land use change and, in general, was higher in disturbed sites. -

Aos Vinte E Dois Dias Do Mês De Fevereiro Do Ano De Dois Mil E Vinte
Ata da Primeira Reunião Ordinária de Posse do Comitê da Bacia Hidrográfica do Tietê Batalha no ano de 2021, de 22 de fevereiro de 2021, resumo publicado no Diário Oficial do Estado em 05/03/2021. Aos vinte e dois dias do mês de fevereiro do ano de dois mil e vinte e um, às 10h, através da plataforma de videoconferência Microsoft Teams, compareceram os membros do Comitê da Bacia Hidrográfica do Tietê Batalha para a realização da 1ª Reunião Ordinária de Posse do CBH/TB. Estiveram presentes cinquenta membros, sendo treze Prefeitos representando o segmento Municípios, com nove titulares, à saber: Vladmir Antonio Adabo (Prefeito Municipal de Borborema), Taís Fernanda Maimoni Contieri Santana (Prefeita Municipal de Cafelândia), Bruno Floriano de Oliveira (Prefeito Municipal de Guaiçara), Vladimir do Carmo Reggiani (Prefeito Municipal de Itápolis), Marcio Perpetuo Augusto (Prefeito Municipal de Marapoama), Cesar Henrique da Cunha Fiala (Prefeito Municipal de Pirajuí), Artur Manoel Nogueira Franco (Prefeito Municipal de Promissão), Eder Ruiz Magalhães de Andrade (Prefeito Municipal de Sabino) e Alcemir Cássio Gréggio (Prefeito Municipal de Urupês); e quatro suplentes, a saber: Antônio Carlos de Mattos Santos (Prefeito Municipal de Dobrada), Cássio Roberto Bertelli (Prefeito Municipal de Elisiário), Sidiomar Ujaque (Prefeito Municipal de Itajobi) e Josemar Francisco de Abreu (Prefeito Municipal de Sales); outras cinco Prefeituras Municipais tiveram representação, sendo elas: Prefeitura Municipal de Bauru, Prefeitura Municipal de Ibirá, Prefeitura -

Relatório Anual De Atividades 16º Legislatura 2017 a 2020
RELATÓRIO ANUAL DE ATIVIDADES 16º LEGISLATURA 2017 A 2020 VEREADOR LUIZ ANTÔNIO GARCIA GUILHEN Presidente da Câmara – Biênio 2017/2018 Igaraçu do Tietê, dezembro de 2017. 1 APRESENTAÇÃO A Câmara da Estância Turística de Igaraçu do Tietê, busca sempre manter a transparência de suas atividades, bem como dos atos dos representantes políticos. Dessa forma o Legislativo igaraçuense mantém em permanente exercício a divulgação de suas ações, de modo que a comunidade local tenha a possibilidade de monitorar todas as atividades desenvolvidas por esta Casa de Leis. MESA DIRETORA ELEITA PARA O BIÊNIO 2017/2018 Presidente Luiz Antônio Garcia Guilhen - PRP Vice-Presidente Geraldo Alves de Oliveira Filho - PSC 1º Secretário Fernando Luiz de Almeida - PRP 2º Secretário Salvador Rodrigues Segura - PSDB VEREADORES Aparecido Jovanir Pena Jr. - PP Elaine Ap. Fernandes Gama - PSDB Hidelbrando Ramos dos Santos - PRTB Genival Donizete Segura - PP Gesner Tadeu Justo - PPS Osvaldo Rogério Varasquim- PMDB Wamberto Picolli - DEM COMISSÕES PERMANENTES – BIÊNIO 2017/2018 COMISSÃO JUSTIÇA E REDAÇÃO PRESIDENTE – APARECIDO JOVANIR PENA JR. MEMBRO – GERALDO ALVES DE OLIVEIRA FILHO MEMBRO – HILDEBRANDO RAMOS DOS SANTOS COMISSÃO FINANÇAS E PRESIDENTE – OSVALDO ROGÉRIO VARASQUIM ORÇAMENTO MEMBRO – GENIVAL DONIZETE SEGURA MEMBRO – GESNER TADEU JUSTO 2 COMISSÃO OBRAS, SERVIÇOS PRESIDENTE – SALVADOR RODRIGUES SEGURA PÚBLICOS E ENTIDADES PRIVADAS MEMBRO – APARECIDO JOVANIR PENA JR. MEMBRO – FERNANDO LUIZ DE ALMEIDA COMISSÃO EDUCAÇÃO, SAÚDE E PRESIDENTE – ELAINE AP. FERNANDES GAMA ASSISTÊNCIA SOCIAL MEMBRO – WAMBERTO PICOLLI MEMBRO – OSVALDO ROGÉRIO VARASQUIM QUADRO DE PESSOAL O quadro geral de pessoal do Legislativo conta atualmente com cinco funcionários efetivos. Servidores Efetivos Função Maria José S. Santos Lupino Diretora Geral da Câmara Luiz Rodolfo Olivato Contador Rosangela Ap. -

Banco Do Brasil ***932551** ANDREIA RASTEIRO LEVINO
Banco do Brasil ***932551** ANDREIA RASTEIRO LEVINO Bady Bassitt Sp Banco do Brasil ***271825** CATIA RENATA OLIVEIRA MARTINS Bady Bassitt Sp Banco do Brasil ***458858** CE LINA MARIA DO CARMO CIENCIA Bady Bassitt Sp Banco do Brasil ***391737** DEBORA CRISTINA LOURENCIM Bady Bassitt Sp Banco do Brasil ***288061** GRAZIELE CRISTINA CLINIO DA SILVA Bady Bassitt Sp Banco do Brasil ***884971** JANAINA DE FATIMA ROSA Bady Bassitt Sp Banco do Brasil ***158643** KEDIMA RODRIGUES DE ANDRADE Bady Bassitt Sp Banco do Brasil ***600031** LUIZ CARLOS DA SILVA Bady Bassitt Sp Banco do Brasil ***798832** MARCIO ELIAS DOS SANTOS Bady Bassitt Sp Banco do Brasil ***982397** MARIA DAS GRACAS DA SILVA Bady Bassitt Sp Banco do Brasil ***036572** MARIA MADALENA SANTANA MORAES Bady Bassitt Sp Banco do Brasil ***818048** NAYLA REGINA GUILHERMITTI BATISTA Bady Bassitt Sp Banco do Brasil ***483745** PATRICIA FERNANDA BARUFI Bady Bassitt Sp Banco do Brasil ***228141** SOELI FATIMA SOLEMAN Bady Bassitt Sp Banco do Brasil ***383493** VALERIA DE FREITAS BARBOSA DA COSTA Bady Bassitt Sp Banco do Brasil ***624021** ABILIO TOMAS Balbinos Sp Banco do Brasil ***061145** AUGUSTO BAZILIO Balbinos Sp Banco do Brasil ***190391** CLAUDIA APARECIDA DE CARVALHO Balbinos Sp Banco do Brasil ***121206** PAULO SERGIOGUANDALIN Balbinos Sp Banco do Brasil ***386731** PRISCILA ZACARIN QUIOVETTO Balsamo Sp Banco do Brasil ***279403** ELENILDO DE ANDRADE CORREA Bananal Sp Banco do Brasil ***570586** ELIANE AVELAR NOGUEIRA Bananal Sp Banco do Brasil ***776834** GILBERTO DE ANDRADE Bananal -

Resultados Dia 18-01-2018
RESULTADOS DIA 18-01-2018 BO Mineiros do Tietê 0 x W Reginópolis BO S.Rita Passa Quatro 18 x 6 Perderneiras BO Piracicaba 18 x 14 Lins BO Dourado 18 x 14 Barra Bonita BO São Carlos 18 x 14 Águas São Pedro BO Pirassununga 0 x W Cafelândia BO S.Rita Passa Quatro 6 x 18 Lençóis Paulista BO Botucatu 18 x 0 Reginópolis BO Bauru 8 x 18 Piracicaba BO Rio das Pedras 6 x 18 São Manuel BO Porto Ferreira 18 x 16 Piratininga BO Dourado 16 x 18 Igaraçu do Tietê BU F Porto Ferreira 2 x 0 São Manuel BU F Bauru W x 0 Jaú BU F Brotas 2 x 1 Águas São Pedro BU F Rio das Pedras 0 x W Dois Córregos BU F Pirassununga 2 x 0 Pederneiras BU F Piracicaba 2 x 0 Itirapina BU F Anhembi 0 x W Promissão BU F São Manuel 0 x 2 Botucatu BU F Jaú 0 x W S.Rita Passa Quatro BU F Águas São Pedro W x 0 Rio das Pedras BU F Dois Córregos 0 x 2 Brotas BU F Pederneiras 2 x 1 Lençóis Paulista BU F Itirapina W0 x WO Anhembi BU F Promissão 0 x 2 Piracicaba BU F Promissão 0 x 2 Piracicaba BU M S.Rita Passa Quatro 2 x 1 Brotas BU M Lins 0 x 2 Pirassununga BU M Botucatu 1 x 2 Getulina BU M Sabino 2 x 0 Porto Ferreira BU M Promissão 2 x 1 Itirapina BU M Jaú 2 x 1 Águas São Pedro BU M Piracicaba 1 x 2 Bauru BU M Brotas 0 x 2 Piratininga BU M Pirassununga 2 x 1 Botucatu BU M Getulina 2 x 1 Lins BU M Porto Ferreira 1 x 2 Promissão BU M Itirapina 0 x W Sabino BU M Águas São Pedro 1 x 2 Piracicaba BU M Bauru 0 x 2 Jaú DO F Piracicaba 4 x 3 Botucatu DO F Brotas 4 x 3 São Manuel DO F Pirassununga 4 x 0 Rio das Pedras DO F Pratânia 2 x 4 Itapui DO F Lençóis Paulista 4 x 2 Bauru DO F Barra Bonita -

Relatório Anual De Atividades 16º Legislatura 2017 a 2020
RELATÓRIO ANUAL DE ATIVIDADES 16º LEGISLATURA 2017 A 2020 VEREADOR DR. APARECIDO JOVANIR PENA JR. Presidente da Câmara – Biênio 2019/2020 Igaraçu do Tietê, dezembro de 2019. 1 APRESENTAÇÃO A Câmara da Estância Turística de Igaraçu do Tietê, busca sempre manter a transparência de suas atividades, bem como dos atos dos representantes políticos. Dessa forma o Legislativo igaraçuense mantém em permanente exercício a divulgação de suas ações, de modo que a comunidade local tenha a possibilidade de monitorar todas as atividades desenvolvidas por esta Casa de Leis. MESA DIRETORA ELEITA PARA O BIÊNIO 2019/2020 Presidente Aparecido Jovanir Pena Jr. - PP Vice-Presidente Elaine Ap. Fernandes Gama - PSDB 1º Secretário Luiz Antônio Garcia Guilhen - PRP 2º Secretário Geraldo Alves de Oliveira Filho - PSC VEREADORES Fernando Luiz de Almeida - PRP Salvador Rodrigues Segura - PSDB Hidelbrando Ramos dos Santos - PRTB Genival Donizete Segura - PP Gesner Tadeu Justo - PPS Osvaldo Rogério Varasquim- PMDB Wamberto Picolli - DEM COMISSÕES PERMANENTES – BIÊNIO 2019/2020 COMISSÃO JUSTIÇA E REDAÇÃO PRESIDENTE – OSVALDO ROGÉRIO VARASQUIM MEMBRO – FERNANDO LUIZ DE ALMEIDA MEMBRO – SALVADOR RODRIGUES SEGURA COMISSÃO FINANÇAS E PRESIDENTE – LUIZ ANTONIO GARCIA GUILHEN ORÇAMENTO MEMBRO – HILDEBRANDO RAMOS DOS SANTOS MEMBRO – OSVALDO ROGÉRIO VARASQUIM COMISSÃO OBRAS, SERVIÇOS PRESIDENTE – GERALDO ALVES DE OLIVEIRA FILHO PÚBLICOS E ENTIDADES PRIVADAS MEMBRO – WAMBERTO PICOLLI MEMBRO – ELAINE AP. FERNANDES GAMA COMISSÃO EDUCAÇÃO, SAÚDE E PRESIDENTE – SALVADOR RODRIGUES SEGURA ASSISTÊNCIA SOCIAL MEMBRO – GESNER TADEU JUSTO MEMBRO – GENIVAL DONIZETE SEGURA 2 QUADRO DE PESSOAL O quadro geral de pessoal do Legislativo conta atualmente com cinco funcionários efetivos. Servidores Efetivos Função Maria José S. Santos Lupino Diretora Geral da Câmara Luiz Rodolfo Olivato Contador Rosangela Ap. -

Escolas Municipais De Educação Básica CIFE
Município de São Bernardo do Campo Secretaria de Educação Lista de EMEBs - Escolas Municipais de Educação Básica CIFE Unidade Educacional Logradouro Bairro CEP Tel Direção C Afonso Monteiro da Cruz - EMEB Rua Valdomiro Luiz,175 Demarchi 09820-340 4347-7586 TEREZA MARIA DE PAULA C Agostinho dos Santos - EMEB Rua Austrália,90 Jardim Santo Ignácio 09861-390 4335-5304 FABIOLA OTTATI I Aldino Pinotti - EMEB Rua dos Vianas,2399 Vila Baeta Neves 09760-510 4332-7537 STELA PEREIRA DA GAMA F Aldino Pinotti, Prefeito - EMEB Rua Antônio Simionato,103 Vila Santa Terezinha 09780-180 4330-2374 FLAVIA MARQUES F Alfredo Scarpelli - EMEB Estrada dos Alvarengas,4090 Assunção 09850-550 4357-5196 CI Alice do Lago Gonçalves Salvador, Professora - EMEB Rua das Rosas,s/n Montanhão 09784-165 4345-1847 FRANCINILDO DE SOUSA BARBOSA I Aluísio de Azevedo - EMEB Rua Cabral da Câmara,57 Jardim Embaré 09895-200 4399-1651 C Alzira Martins de Mendonça - EMEB Rua Padre Léo Comissari,550 Ferrazópolis 09791-006 I Ana Henriqueta Clark Marim, Professora - EMEB Rua Sabino Demarchi,50 Jardim São José 09762-040 4122-1506 JANE BEZERRA DA SILVA I Ana Maria Poppovic - EMEB Rua Carlos Wunderlick,100 Dos Casa 09840-460 4109-6498 CIRLENE LOURENCO CAPUTO F André Ferreira, Professor - EMEB Rua Regente Lima e Silva,301 Ferrazópolis 09781-130 4127-0232 ANA PAULA VILAS BOAS CAETANO F Ângelo Ceroni, Padre - EMEB Alameda Dom Pedro de Alcântara,805 Jardim Nova Petrópolis 09771-281 4330-8613 CARMEN LUCIA LOPES CRETUCCI CI Anísio Teixeira - EMEB Rua Doutor Francisco da Silva Prado,132 Vila Flórida -

DIÁRIO OFICIAL MUNICÍPIO DE PAULICÉIA Conforme Lei Municipal Nº 86/2019, De 14 De Março De 2019
DIÁRIO OFICIAL MUNICÍPIO DE PAULICÉIA Conforme Lei Municipal nº 86/2019, de 14 de março de 2019 www.pauliceia.sp.gov.br | www.imprensaoficialmunicipal.com.br/pauliceia Quinta-feira, 01 de outubro de 2020 Ano II | Edição nº 354 Página 1 de 69 EXPEDIENTE SUMÁRIO O Diário Oficial do Município de Paulicéia, veiculado PODER EXECUTIVO DE PAULICÉIA 2 exclusivamente na forma eletrônica, é uma publicação Atos de Pessoal 2 das entidades da Administração Direta e Indireta deste Outros atos 2 Município, sendo referidas entidades inteiramente responsáveis pelo conteúdo aqui publicado. ACERVO As edições do Diário Oficial Eletrônico de Paulicéia poderão ser consultadas através da internet, por meio do seguinte endereço eletrônico: www.pauliceia.sp.gov.br Para pesquisa por qualquer termo e utilização de filtros, acesse www.imprensaoficialmunicipal.com.br/pauliceia As consultas e pesquisas são de acesso gratuito e independente de qualquer cadastro. ENTIDADES Prefeitura Municipal de Paulicéia CNPJ 44.918.928/0001-25 Avenida Paulista, 1649 Telefone: (18) 3876-1240 Site: www.pauliceia.sp.gov.br Diário: www.imprensaoficialmunicipal.com.br/pauliceia Câmara Municipal de Paulicéia CNPJ 53.306.932/0001-23 Av. Paulista, 2260 - Aquarius I Telefone: (18) 3876-1241 Site: www.camarapauliceia.sp.gov.br Diário Oficial Assinado Eletronicamente com Certificado Padrão ICPBrasil, em conformidade com a MP nº 2.200-2, de 2001 O Município de Paulicéia garante a autenticidade deste documento, desde que visualizado através do site www.pauliceia.sp.gov.br Compilado e também disponível -
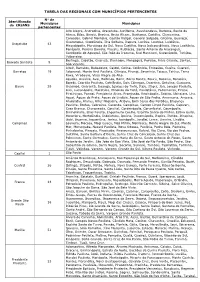
Tabela Das Regionais Com Municípios Pertencentes
TABELA DAS REGIONAIS COM MUNICÍPIOS PERTENCENTES Nº de Identificação Municípios Municípios da CRSANS pertencentes Alto Alegre, Andradina, Araçatuba, Auriflama, Avanhandava, Barbosa, Bento de Abreu, Bilac, Birigüi, Braúna, Brejo Alegre, Buritama, Castilho, Clementina, Coroados, Gabriel Monteiro, Gastão Vidigal, General Salgado, Glicério, Guaraçaí, Guararapes, Guzolândia, Ilha Solteira, Itapura, Lavínia, Lourdes, Luiziânia, Araçatuba 43 Mirandópolis, Murutinga do Sul, Nova Castilho, Nova Independência, Nova Luzitânia, Penápolis, Pereira Barreto, Piacatu, Rubiácea, Santo Antonio do Aracanguá, Santópolis do Aguapeí, São João de Iracema, Sud Mennucci, Suzanápolis, Turiúba, Valparaíso. Bertioga, Cubatão, Guarujá, Itanhaém, Mongaguá, Peruíbe, Praia Grande, Santos, Baixada Santista 9 São Vicente. Altair, Barretos, Bebedouro, Cajobi, Colina, Colômbia, Embaúba, Guaíra, Guaraci, Barretos 19 Jaborandi, Monte Azul Paulista, Olímpia, Pirangi, Severínia, Taiaçu, Taiúva, Terra Roxa, Viradouro, Vista Alegre do Alto. Agudos, Arealva, Avaí, Balbinos, Bariri, Barra Bonita, Bauru, Bocaina, Boracéia, Borebi, Cabrália Paulista, Cafelândia, Dois Córregos, Duartina, Getulina, Guaiçara, Bauru 39 Guaimbê, Guarantã, Iacanga, Igaraçu do Tietê, Itaju, Itapuí, Jaú, Lençóis Paulista, Lins, Lucianópolis, Macatuba, Mineiros do Tietê, Paulistânia, Pederneiras, Pirajuí, Piratininga, Pongaí, Presidente Alves, Promissão, Reginópolis, Sabino, Ubirajara, Uru. Aguaí, Águas da Prata, Águas de Lindóia, Águas de São Pedro, Americana, Amparo, Analândia, Araras, Artur Nogueira, Atibaia,