Introduction to Pseudopotentials and Electronic Structure Philip B
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Simplified Hartree-Fock Computations on Second-Row Atoms
Simplified Hartree-Fock Computations on Second-Row Atoms S. M. Blinder Wolfram Research, Inc. Champaign, IL 61820, USA May 18, 2021 Modern computational quantum chemistry has developed to a large extent beginning with applications of the Hartree-Fock method to atoms and molecules[1]. Density-functional methods have now largely supplanted conventional Hartree-Fock computations in current applications of computational chemistry. Still, from a pedagogical point of view, an un- derstanding of the original methods remains a necessary preliminary. However, since more elaborate Hartree-Fock computations are now no longer needed, it will suffice to consider a computationally simplified application of the method[2]. Accordingly, we will represent the many-electron wavefunction by a single closed-shell Slater determinant and use basis functions which are simple Slater-type orbitals. This is in contrast to the more elaborate Hartree-Fock computations, which might use multi-determinant wavefunctions and double- zeta basis functions. All of the symbolic and numerical operations were carried out using Mathematica. We consider Hartree-Fock computations on the ground states of the second-row atoms He through Ne, Z = 2 to 10, using the simplest set of orthonormalized 1s, 2s and 2p orbital functions: s α3=2 3β5 α + β = p e−αr; = 1 − r e−βr; 1s π 2s π(α2 − αβ + β2) 3 5=2 γ −γr 2pfx;y;zg = p re fsin θ cos φ, sin θ sin φ, cos θg: (1) arXiv:2105.07018v1 [quant-ph] 14 May 2021 π An orbital function multiplied by a spin function α or β is known as a spinorbital, a product of the form ( α φ(x) = (r) : (2) β 1 A simple representation of a many-electron atom is given by a Slater determinant con- structed from N occupied spin-orbitals: φa(1) φb(1) : : : φn(1) 1 φa(2) φb(2) : : : φn(2) Ψ(1;:::;N) = p ; (3) . -

A Self-Interaction-Free Local Hybrid Functional: Accurate Binding
A self-interaction-free local hybrid functional: accurate binding energies vis-`a-vis accurate ionization potentials from Kohn-Sham eigenvalues Tobias Schmidt,1, ∗ Eli Kraisler,2, ∗ Adi Makmal,2, † Leeor Kronik,2 and Stephan K¨ummel1 1Theoretical Physics IV, University of Bayreuth, 95440 Bayreuth, Germany 2Department of Materials and Interfaces, Weizmann Institute of Science, Rehovoth 76100,Israel (Dated: September 12, 2018) We present and test a new approximation for the exchange-correlation (xc) energy of Kohn- Sham density functional theory. It combines exact exchange with a compatible non-local correlation functional. The functional is by construction free of one-electron self-interaction, respects constraints derived from uniform coordinate scaling, and has the correct asymptotic behavior of the xc energy density. It contains one parameter that is not determined ab initio. We investigate whether it is possible to construct a functional that yields accurate binding energies and affords other advantages, specifically Kohn-Sham eigenvalues that reliably reflect ionization potentials. Tests for a set of atoms and small molecules show that within our local-hybrid form accurate binding energies can be achieved by proper optimization of the free parameter in our functional, along with an improvement in dissociation energy curves and in Kohn-Sham eigenvalues. However, the correspondence of the latter to experimental ionization potentials is not yet satisfactory, and if we choose to optimize their prediction, a rather different value of the functional’s parameter is obtained. We put this finding in a larger context by discussing similar observations for other functionals and possible directions for further functional development that our findings suggest. -

Account 0103 - 5053 $6.00+0.00Ac Carbocations on Zeolites
J. Braz. Chem. Soc., Vol. 22, No. 7, 1197-1205, 2011. Printed in Brazil - ©2011 Sociedade Brasileira de Química Account 0103 - 5053 $6.00+0.00Ac Carbocations on Zeolites. Quo Vadis? Claudio J. A. Mota* and Nilton Rosenbach Jr.# Instituto de Química and INCT de Energia e Ambiente, Universidade Federal do Rio de Janeiro, Cidade Universitária, CT Bloco A, 21941-909 Rio de Janeiro-RJ, Brazil A natureza dos carbocátions adsorvidos na superfície da zeólita é discutida neste artigo, destacando estudos experimentais e teóricos. A adsorção de halogenetos de alquila sobre zeólitas trocadas com metais tem sido usada para estudar o equilíbrio entre alcóxidos, que são espécies covalentes, e carbocátions, que têm natureza iônica. Cálculos teóricos indicam que os carbocátions são mínimos (intermediários) na superfície de energia potencial e estabilizados por ligações hidrogênio com os átomos de oxigênio da estrutura zeolítica. Os resultados indicam que zeólitas se comportam como solventes sólidos, estabilizando a formação de espécies iônicas. The nature of carbocations on the zeolite surface is discussed in this account, highlighting experimental and theoretical studies. The adsorption of alkylhalides over metal-exchanged zeolites has been used to study the equilibrium between covalent alkoxides and ionic carbocations. Theoretical calculations indicated that the carbocations are minima (intermediates) on the potential energy surface and stabilized by hydrogen bonds with the framework oxygen atoms. The results indicate that zeolites behave like solid solvents, stabilizing the formation of ionic species. Keywords: zeolites, carbocations, alkoxides, catalysis, acidity 1. Introduction Thus, NH4Y can be prepared by exchange of the NaY with ammonium salt solutions. An acidic material can Zeolites are crystalline aluminosilicates with pores of be obtained by calcination of the ammonium-exchanged molecular dimensions. -

The Empirical Pseudopotential Method
The empirical pseudopotential method Richard Tran PID: A1070602 1 Background In 1934, Fermi [1] proposed a method to describe high lying atomic states by replacing the complex effects of the core electrons with an effective potential described by the pseudo-wavefunctions of the valence electrons. Shortly after, this pseudopotential (PSP), was realized by Hellmann [2] who implemented it to describe the energy levels of alkali atoms. Despite his initial success, further exploration of the PSP and its application in efficiently solving the Hamiltonian of periodic systems did not reach its zenith until the middle of the twenti- eth century when Herring [3] proposed the orthogonalized plane wave (OPW) method to calculate the wave functions of metals and semiconductors. Using the OPW, Phillips and Kleinman [4] showed that the orthogonality of the va- lence electrons’ wave functions relative to that of the core electrons leads to a cancellation of the attractive and repulsive potentials of the core electrons (with the repulsive potentials being approximated either analytically or empirically), allowing for a rapid convergence of the OPW calculations. Subsequently in the 60s, Marvin Cohen expanded and improved upon the pseudopotential method by using the crystalline energy levels of several semicon- ductor materials [6, 7, 8] to empirically obtain the potentials needed to compute the atomic wave functions, developing what is now known as the empirical pseu- dopotential method (EPM). Although more advanced PSPs such as the ab-initio pseudopotentials exist, the EPM still provides an incredibly accurate method for calculating optical properties and band structures, especially for metals and semiconductors, with a less computationally taxing method. -

ACF NATIONALS 2018 ROUND 1 PRELIMS 1 Packet by OXFORD + DUKE
ACF NATIONALS 2018 ROUND 1 PRELIMS 1 packet by OXFORD + DUKE authors Oxford: Daoud Jackson, George Charlson, Jacob Robertson, Freddy Potts, Chris Stern Duke: Ryan Humphrey, Gabe Guedes, Lucian Li, Annabelle Yang ACF Nationals 2018 | Packet: Oxford + Duke | Page 1 Editors: Jordan Brownstein, Andrew Hart, Stephen Liu, Aaron Rosenberg, Andrew Wang, Ryan Westbrook Tossups 1. ARCIMBOLDO (ar-chim-BOL-do) and the auto-rickshaw platform process data from this technique. The sphere-of- influence algorithm for this technique helps differentiate solvents and the target molecule, and like the free lunch algorithm, is found in the SHELXE (“shell X-E”) package. Data from this technique can be more easily processed by repeating data collection after either using a laser to induce radiation damage in the sample, or soaking the sample in a heavy metal compound. This is the most prominent technique for which Rigaku produces instruments, including the MiniFlex. MAD, SAD, and isomorphous replacement are methods used in this technique to solve the phase problem. The systematic absences from this technique are used to help find the space group of the analyte, which must be determined in order to solve the final structure. For 10 points, name this technique that uses Bragg’s law to determine the structure of a crystal. ANSWER: x-ray diffraction [accept x-ray crystallography or XRD] 2. Five years after this man’s death, his diaries were collected by the manager of the Pomeranian Mortgage Bank, including correspondences produced while he lived with his former lieutenant Wilhelm Junker. This man’s peaceful meeting with Omukama Kabalega (oh-moo-KAH-mah kah-bah-LEH-gah) is commemorated by a cone-shaped structure at the Mparo Royal Tombs. -
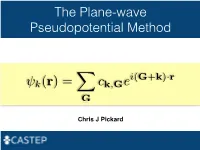
The Plane-Wave Pseudopotential Method
The Plane-wave Pseudopotential Method i(G+k) r k(r)= ck,Ge · XG Chris J Pickard Electrons in a Solid Nearly Free Electrons Nearly Free Electrons Nearly Free Electrons Electronic Structures Methods Empirical Pseudopotentials Ab Initio Pseudopotentials Ab Initio Pseudopotentials Ab Initio Pseudopotentials Ab Initio Pseudopotentials Ultrasoft Pseudopotentials (Vanderbilt, 1990) Projector Augmented Waves Deriving the Pseudo Hamiltonian PAW vs PAW C. G. van de Walle and P. E. Bloechl 1993 (I) PAW to calculate all electron properties from PSP calculations" P. E. Bloechl 1994 (II)! The PAW electronic structure method" " The two are linked, but should not be confused References David Vanderbilt, Soft self-consistent pseudopotentials in a generalised eigenvalue formalism, PRB 1990 41 7892" Kari Laasonen et al, Car-Parinello molecular dynamics with Vanderbilt ultrasoft pseudopotentials, PRB 1993 47 10142" P.E. Bloechl, Projector augmented-wave method, PRB 1994 50 17953" G. Kresse and D. Joubert, From ultrasoft pseudopotentials to the projector augmented-wave method, PRB 1999 59 1758 On-the-fly Pseudopotentials - All quantities for PAW (I) reconstruction available" - Allows automatic consistency of the potentials with functionals" - Even fewer input files required" - The code in CASTEP is a cut down (fast) pseudopotential code" - There are internal databases" - Other generation codes (Vanderbilt’s original, OPIUM, LD1) exist It all works! Kohn-Sham Equations Being (Self) Consistent Eigenproblem in a Basis Just a Few Atoms In a Crystal Plane-waves -

Core Electron Binding Energies in Heavy Atoms
l~m;~, Vol. 21, No. 2, August 1983, pp. 103-110. © Printed in India. Core electron binding energies in heavy atoms M P DAS Department of Physics, Sambalpur University,Jyoti Vihar, Sambalpur 768 017, India MS received 8 October 1982; revised 26 April 1983 Abstract. Inner shell binding of electrons in heavy atoms is studied through the relativistic density functional theory in which many electron interactions are treated in a local density approximation. By using this theory and the A scF procedure binding energies of several core electrons of mercury atom are calculatedin the frozen and relaxed configurations. The results are compared with those carried out by the non-local Dirac-Fock Scheme. K-shellbinding energies of several closed shell atoms are calculated by using the Kolm-Shamand the relativisticexchange potentials. The results are discussed and the discrepancies in our local densityresults, when compared with experimental values, may be attributed to the non-localityand to the many-body effects. Keywords. Atomic structure; binding ener~; relaxed orbitals; density functional; Breit interaction. 1. Introduction Calculations on the electronic structure of heavy atoms based on the relativistic theory, such as multi-oonfigurational Dirac-Fock (MeDF) method (Desolaux 1980) are in reasonably good agreement with experimental findings. This theory employs one- electron Dirao Hamiltonian that contains the kinematics of the electrons and their interactions with the classical nuclear field. The electron-electron interaction is then added in two parts. The first part is treated in ~e Hartree-Fock sense by applying variational principle to a many-electron wavefunction as an antisymmctric product of one-electron orbitals. -

Introduction to DFT and the Plane-Wave Pseudopotential Method
Introduction to DFT and the plane-wave pseudopotential method Keith Refson STFC Rutherford Appleton Laboratory Chilton, Didcot, OXON OX11 0QX 23 Apr 2014 Parallel Materials Modelling Packages @ EPCC 1 / 55 Introduction Synopsis Motivation Some ab initio codes Quantum-mechanical approaches Density Functional Theory Electronic Structure of Condensed Phases Total-energy calculations Introduction Basis sets Plane-waves and Pseudopotentials How to solve the equations Parallel Materials Modelling Packages @ EPCC 2 / 55 Synopsis Introduction A guided tour inside the “black box” of ab-initio simulation. Synopsis • Motivation • The rise of quantum-mechanical simulations. Some ab initio codes Wavefunction-based theory • Density-functional theory (DFT) Quantum-mechanical • approaches Quantum theory in periodic boundaries • Plane-wave and other basis sets Density Functional • Theory SCF solvers • Molecular Dynamics Electronic Structure of Condensed Phases Recommended Reading and Further Study Total-energy calculations • Basis sets Jorge Kohanoff Electronic Structure Calculations for Solids and Molecules, Plane-waves and Theory and Computational Methods, Cambridge, ISBN-13: 9780521815918 Pseudopotentials • Dominik Marx, J¨urg Hutter Ab Initio Molecular Dynamics: Basic Theory and How to solve the Advanced Methods Cambridge University Press, ISBN: 0521898633 equations • Richard M. Martin Electronic Structure: Basic Theory and Practical Methods: Basic Theory and Practical Density Functional Approaches Vol 1 Cambridge University Press, ISBN: 0521782856 -

The Plane-Wave Pseudopotential Method
The Plane-Wave Pseudopotential Method Eckhard Pehlke, Institut für Laser- und Plasmaphysik, Universität Essen, 45117 Essen, Germany. Starting Point: Electronic structure problem from physics, chemistry, materials science, geology, biology, ... which can be solved by total-energy calculations. Topics of this (i) how to get rid of the "core electrons": talk: the pseudopotential concept (ii) the plane-wave basis-set and its advantages (iii) supercells, Bloch theorem and Brillouin zone integrals choices we have... Treatment of electron-electron interaction and decisions to make... Hartree-Fock (HF) ¿ ÒÌ Ò· Ú ´ÖµÒ´Öµ Ö Ú ¼ ¼ ½ Ò´ÖµÒ´Ö µ ¿ ¿ ¼ · Ö Ö · Ò Configuration-Interaction (CI) ¼ ¾ Ö Ö Quantum Monte-Carlo (QMC) ÑÒ Ò ¼ Ú Ê ¿ Ò´Ö µ ÖÆ Density-Functional Theory (DFT) ¼ ½ Ò´Ö µ ¾ ¿ ¼ ´ Ö · Ú ´Öµ· Ö · Ú ´Öµµ ´Öµ ´Öµ ¼ ¾ Ö Ö Æ Ò ¾ Ò´Öµ ´Öµ Ú ´Öµ Æ´Öµ Problem: Approximation to XC functional. decisions to make... Simulation of Atomic Geometries Example: chemisorption site & energy of a particular atom on a surface = ? How to simulate adsorption geometry? single molecule or cluster Use Bloch theorem. periodically repeated supercell, slab-geometry Efficient Brillouin zone integration schemes. true half-space geometry, Green-function methods decisions to make... Basis Set to Expand Wave-Functions ½ ¡Ê ´Øµ ´ Öµ Ù ´Ö Ê µ Ô Æ linear combination of Ê atomic orbitals (LCAO) ´ Öµ ´µ ´ Öµ simple, unbiased, ½ ´·µ¡Ö ´ Öµ ´ · µ plane waves (PW) Ô independent of ª atomic positions augmented plane waves . (APW) etc. etc. I. The Pseudopotential Concept Core-States and Chemical Bonding? Validity of the Frozen-Core Approximation U. von Barth, C.D. -

Car-Parrinello Molecular Dynamics
CPMD Car-Parrinello Molecular Dynamics An ab initio Electronic Structure and Molecular Dynamics Program The CPMD consortium WWW: http://www.cpmd.org/ Mailing list: [email protected] E-mail: [email protected] January 29, 2019 Send comments and bug reports to [email protected] This manual is for CPMD version 4.3.0 CPMD 4.3.0 January 29, 2019 Contents I Overview3 1 About this manual3 2 Citation 3 3 Important constants and conversion factors3 4 Recommendations for further reading4 5 History 5 5.1 CPMD Version 1.....................................5 5.2 CPMD Version 2.....................................5 5.2.1 Version 2.0....................................5 5.2.2 Version 2.5....................................5 5.3 CPMD Version 3.....................................5 5.3.1 Version 3.0....................................5 5.3.2 Version 3.1....................................5 5.3.3 Version 3.2....................................5 5.3.4 Version 3.3....................................6 5.3.5 Version 3.4....................................6 5.3.6 Version 3.5....................................6 5.3.7 Version 3.6....................................6 5.3.8 Version 3.7....................................6 5.3.9 Version 3.8....................................6 5.3.10 Version 3.9....................................6 5.3.11 Version 3.10....................................7 5.3.12 Version 3.11....................................7 5.3.13 Version 3.12....................................7 5.3.14 Version 3.13....................................7 5.3.15 Version 3.14....................................7 5.3.16 Version 3.15....................................8 5.3.17 Version 3.17....................................8 5.4 CPMD Version 4.....................................8 5.4.1 Version 4.0....................................8 5.4.2 Version 4.1....................................8 5.4.3 Version 4.3....................................9 6 Installation 10 7 Running CPMD 11 8 Files 12 II Reference Manual 15 9 Input File Reference 15 9.1 Basic rules........................................ -

New Method for Measuring Angle-Resolved Phases in Photoemission
PHYSICAL REVIEW X 10, 031070 (2020) New Method for Measuring Angle-Resolved Phases in Photoemission Daehyun You ,1 Kiyoshi Ueda,1,* Elena V. Gryzlova ,2 Alexei N. Grum-Grzhimailo ,2 Maria M. Popova ,2,3 Ekaterina I. Staroselskaya,3 Oyunbileg Tugs,4 Yuki Orimo ,4 Takeshi Sato ,4,5,6 Kenichi L. Ishikawa ,4,5,6 Paolo Antonio Carpeggiani ,7 Tamás Csizmadia,8 Miklós Füle,8 Giuseppe Sansone ,9 Praveen Kumar Maroju,9 Alessandro D’Elia ,10,11 Tommaso Mazza,12 Michael Meyer ,12 Carlo Callegari ,13 Michele Di Fraia,13 Oksana Plekan ,13 Robert Richter,13 Luca Giannessi,13,14 Enrico Allaria,13 Giovanni De Ninno,13,15 Mauro Trovò,13 ‡ Laura Badano,13 Bruno Diviacco ,13 Giulio Gaio,13 David Gauthier,13, Najmeh Mirian,13 Giuseppe Penco,13 † Primož Rebernik Ribič ,13,15 Simone Spampinati,13 Carlo Spezzani,13 and Kevin C. Prince 13,16, 1Institute of Multidisciplinary Research for Advanced Materials, Tohoku University, Sendai 980-8577, Japan 2Skobeltsyn Institute of Nuclear Physics, Lomonosov Moscow State University, Moscow 119991, Russia 3Faculty of Physics, Lomonosov Moscow State University, Moscow 119991, Russia 4Department of Nuclear Engineering and Management, Graduate School of Engineering, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8656, Japan 5Photon Science Center, Graduate School of Engineering, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8656, Japan 6Research Institute for Photon Science and Laser Technology, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-0033, Japan 7Institut für Photonik, Technische -

Qbox (Qb@Ll Branch)
Qbox (qb@ll branch) Summary Version 1.2 Purpose of Benchmark Qbox is a first-principles molecular dynamics code used to compute the properties of materials directly from the underlying physics equations. Density Functional Theory is used to provide a quantum-mechanical description of chemically-active electrons and nonlocal norm-conserving pseudopotentials are used to represent ions and core electrons. The method has O(N3) computational complexity, where N is the total number of valence electrons in the system. Characteristics of Benchmark The computational workload of a Qbox run is split between parallel dense linear algebra, carried out by the ScaLAPACK library, and a custom 3D Fast Fourier Transform. The primary data structure, the electronic wavefunction, is distributed across a 2D MPI process grid. The shape of the process grid is defined by the nrowmax variable set in the Qbox input file, which sets the number of process rows. Parallel performance can vary significantly with process grid shape, although past experience has shown that highly rectangular process grids (e.g. 2048 x 64) give the best performance. The majority of the run time is spent in matrix multiplication, Gram-Schmidt orthogonalization and parallel FFTs. Several ScaLAPACK routines do not scale well beyond 100k+ MPI tasks, so threading can be used to run on more cores. The parallel FFT and a few other loops are threaded in Qbox with OpenMP, but most of the threading speedup comes from the use of threaded linear algebra kernels (blas, lapack, ESSL). Qbox requires a minimum of 1GB of main memory per MPI task. Mechanics of Building Benchmark To compile Qbox: 1.