New Insights Into the Mammalian Egg Zona Pellucida
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

In Hardening of the Zona Pellucida K
Disulfide formation in bovine zona pellucida glycoproteins during fertilization: evidence for the involvement of cystine cross-linkages in hardening of the zona pellucida K. Kwamoto, K. Ikeda, N. Yonezawa, S. Noguchi, K. Kudo, S. Hamano, M. Kuwayama and M. Nakano department ofChemistry, Faculty ofScience and 2Graduate School ofScience and Technology, Chiba University, 1-33 Yayoi-cho, Inage-ku, Chiba 263-8522, Japan; and3Animal Bio-Technology Center, Livestock Improvement Association, Tokyo, Japan The time for solubilization of the bovine zona pellucida in a hypotonic buffer containing 5% (v/v) \g=b\-mercaptoethanoland 7 mol urea l\m=-\1 increased by 10% after fertilization. Coupling with a specific fluorescent thiol probe, monobromobimane (mBBr), was markedly greater in the zona pellucida of ovarian eggs compared with fertilized eggs, indicating that the cysteine residues in the zona pellucida of unfertilized eggs are oxidized to cystines during fertilization. After endo-\g=b\-galactosidasedigestion to remove N-acetyllactosamine repeats of the carbohydrate chains, three zona pellucida glycoproteins (ZPA, ZPB and ZPC) coupled with the fluorescent bimane groups were fractionated efficiently by reverse-phase HPLC. Estimation of bimane groups in the three components and SDS-PAGE revealed that intramolecular disulfide bonds in ZPA and intra- and intermolecular disulfide bonds in ZPB were formed during fertilization, but oxidation of cysteine residues in ZPC was low. Specific proteolysis of ZPA during fertilization was also observed. These results indicate that the formation of disulfide linkages together with specific proteolysis result in the construction of a rigid zona pellucida structure, which is responsible for hardening of the zona pellucida. Introduction cross-linkages between tyrosine residues of the zona pellucida proteins formed by ovoperoxidase caused the The zona is one of the two sites at which pellucida In contrast to sea urchins (Foerder and is blocked and hardening. -

Chapter 28 *Lecture Powepoint
Chapter 28 *Lecture PowePoint The Female Reproductive System *See separate FlexArt PowerPoint slides for all figures and tables preinserted into PowerPoint without notes. Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Introduction • The female reproductive system is more complex than the male system because it serves more purposes – Produces and delivers gametes – Provides nutrition and safe harbor for fetal development – Gives birth – Nourishes infant • Female system is more cyclic, and the hormones are secreted in a more complex sequence than the relatively steady secretion in the male 28-2 Sexual Differentiation • The two sexes indistinguishable for first 8 to 10 weeks of development • Female reproductive tract develops from the paramesonephric ducts – Not because of the positive action of any hormone – Because of the absence of testosterone and müllerian-inhibiting factor (MIF) 28-3 Reproductive Anatomy • Expected Learning Outcomes – Describe the structure of the ovary – Trace the female reproductive tract and describe the gross anatomy and histology of each organ – Identify the ligaments that support the female reproductive organs – Describe the blood supply to the female reproductive tract – Identify the external genitalia of the female – Describe the structure of the nonlactating breast 28-4 Sexual Differentiation • Without testosterone: – Causes mesonephric ducts to degenerate – Genital tubercle becomes the glans clitoris – Urogenital folds become the labia minora – Labioscrotal folds -
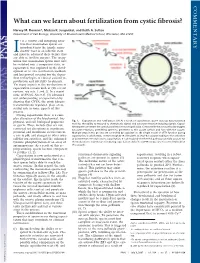
What Can We Learn About Fertilization from Cystic Fibrosis?
COMMENTARY What can we learn about fertilization from cystic fibrosis? Harvey M. Florman*, Melissa K. Jungnickel, and Keith A. Sutton Department of Cell Biology, University of Massachusetts Medical School, Worcester, MA 01655 t is a curious and intriguing situa- tion that mammalian sperm are introduced into the female repro- ductive tract in an infertile state Iand must be educated there before they are able to fertilize oocytes. The recog- nition that mammalian sperm must first be switched into a competent state, or capacitated, was exploited in the devel- opment of in vitro fertilization methods and has proved essential for the depen- dent technologies of clinical assisted re- production and infertility treatments. Yet many aspects of the mechanisms of capacitation remain unclear (for recent reviews, see refs. 1 and 2). In a recent issue of PNAS, Xu et al. (3) advanced our understanding of capacitation by showing that CFTR, the cystic fibrosis transmembrane regulator, plays an es- sential role in some aspects of this process. During capacitation there is a com- plex alteration of the biochemical, bio- physical, and cell biological properties Fig. 1. Capacitation and fertilization. (A) As a result of capacitation, sperm develop hyperactivated motility, the ability to respond to chemotactic signals and acrosome reaction-inducing signals. Capaci- of sperm. These include (but are not tated sperm penetrate the cumulus and reach the zona pellucida. Contact with the zona pellucida triggers restricted to) alterations in membrane acrosome reactions, permitting sperm to penetrate to the oocyte surface and fuse with the oocyte. potential and membrane sterol content, Multiple steps in this process are controlled by capacitation. -

Quaternary Murid Rodents of Timor Part I: New Material of Coryphomys Buehleri Schaub, 1937, and Description of a Second Species of the Genus
QUATERNARY MURID RODENTS OF TIMOR PART I: NEW MATERIAL OF CORYPHOMYS BUEHLERI SCHAUB, 1937, AND DESCRIPTION OF A SECOND SPECIES OF THE GENUS K. P. APLIN Australian National Wildlife Collection, CSIRO Division of Sustainable Ecosystems, Canberra and Division of Vertebrate Zoology (Mammalogy) American Museum of Natural History ([email protected]) K. M. HELGEN Department of Vertebrate Zoology National Museum of Natural History Smithsonian Institution, Washington and Division of Vertebrate Zoology (Mammalogy) American Museum of Natural History ([email protected]) BULLETIN OF THE AMERICAN MUSEUM OF NATURAL HISTORY Number 341, 80 pp., 21 figures, 4 tables Issued July 21, 2010 Copyright E American Museum of Natural History 2010 ISSN 0003-0090 CONTENTS Abstract.......................................................... 3 Introduction . ...................................................... 3 The environmental context ........................................... 5 Materialsandmethods.............................................. 7 Systematics....................................................... 11 Coryphomys Schaub, 1937 ........................................... 11 Coryphomys buehleri Schaub, 1937 . ................................... 12 Extended description of Coryphomys buehleri............................ 12 Coryphomys musseri, sp.nov.......................................... 25 Description.................................................... 26 Coryphomys, sp.indet.............................................. 34 Discussion . .................................................... -

Bull Sperm Capacitation Is Accompanied by Redox Modifications of Proteins
International Journal of Molecular Sciences Article Bull Sperm Capacitation Is Accompanied by Redox Modifications of Proteins Agnieszka Mostek *, Anna Janta , Anna Majewska and Andrzej Ciereszko Department of Gamete and Embryo Biology, Institute of Animal Reproduction and Food Research of Polish Academy of Sciences, 10-748 Olsztyn, Poland; [email protected] (A.J.); [email protected] (A.M.); [email protected] (A.C.) * Correspondence: [email protected]; Tel.: +48-89-5393134 Abstract: The ability to fertilise an egg is acquired by the mammalian sperm during the complex biochemical process called capacitation. Capacitation is accompanied by the production of reactive oxygen species (ROS), but the mechanism of redox regulation during capacitation has not been elucidated. This study aimed to verify whether capacitation coincides with reversible oxidative post-translational modifications of proteins (oxPTMs). Flow cytometry, fluorescence microscopy and Western blot analyses were used to verify the sperm capacitation process. A fluorescent gel-based redox proteomic approach allowed us to observe changes in the level of reversible oxPTMs manifested by the reduction or oxidation of susceptible cysteines in sperm proteins. Sperm capacitation was accompanied with redox modifications of 48 protein spots corresponding to 22 proteins involved in the production of ROS (SOD, DLD), playing a role in downstream redox signal transfer (GAPDHS and GST) related to the cAMP/PKA pathway (ROPN1L, SPA17), acrosome exocytosis (ACRB, sperm acrosome associated protein 9, IZUMO4), actin polymerisation (CAPZB) and hyperactivation Citation: Mostek, A.; Janta, A.; (TUBB4B, TUB1A). The results demonstrated that sperm capacitation is accompanied by altered Majewska, A.; Ciereszko, A. -

Formation of the Hamster Zona Pellucida in Relation to Ovarian Differentiation and Follicular Growth M
Formation of the hamster zona pellucida in relation to ovarian differentiation and follicular growth M. C. L\l=e'\veill\l=e'\,K. D. Roberts, S. Chevalier, A. Chapdelaine and G. Bleau Departments of Obstetrics and Gynecology, Biochemistry and Medicine, University of Montreal and Maisonneuve-Rosemont Research Center, Montreal, Quebec, Canada H1T 2M4 Summary. Using an immunofluorescence technique on ovarian sections, zona\x=req-\ immunoreactive components were detected in the cytoplasm of the oocyte from the beginning of its growth, when it is surrounded by only a thin squamous follicular cell layer, up to the end of its growth. In parallel with oocyte growth, the staining intensity decreased in the ooplasm. No staining was observed in the cytoplasm of the granulosa cells during normal follicular development in adult cyclic females. However, staining of the granulosa cells was observed at some stages of follicular development in immature females. This staining was especially evident in the ovaries of immature females (22 or 26 days old) stimulated with PMSG. In addition, the staining of the granulosa cells was consistently observed in ovaries showing an abnormal histology. Increased staining of the zona at its outer and inner regions could be distinguished in normal follicles, but when staining occurred on the granulosa cells no such pattern was observed over the zona matrix. These studies indicate that the oocyte itself but not the granulosa cells elaborates the native immunogenic material of the zona pellucida. The administration of PMSG at particular stages of ovarian differentiation interferes with follicular development leading to an abnormal extracellular assembly of the zona and its degradation (phagocytosis) by the surrounding granulosa cells. -

Changes in the Composition of the Hamster Zona Pellucida After Fertilization in Vivo but Not in Vitro C
Changes in the composition of the hamster zona pellucida after fertilization in vivo but not in vitro C. R. Brown, N. Clarke, M. Aiken and B. D. Bavister Institute of Animal Physiology and Genetics Research, Babraham Hall, Cambridge CB2 4AT, UK; and * Department ofVeterinary Science, University of Wisconsin, Madison, WI53706, USA Summary. Hamster zonae pellucidae were obtained from follicular oocytes, super- ovulated eggs, and eggs fertilized in vivo or in vitro. Zonae were labelled with N-succinimidyl-3(4-hydroxy,5-[125I]iodophenyl)propionate, and compared on single\x=req-\ and two-dimensional SDS-PAGE. Single-dimensional electrophoresis showed consider- able differences between zona categories in the amount of label that they incorporated; follicular zonae incorporated the least label and zonae from eggs fertilized in vivo the most. On two-dimensional electrophoresis, polypeptides from 3 of the 4 zona categories migrated into 4 major groups: two of these groups each with Mr 150 000\p=n-\250000 were within the Mr range of ZP1, and two others, at Mr90 000 and 55 000, appeared to be analogous to ZP2 and ZP3, respectively. The fourth zona category (zonae from eggs fertilized in vivo) showed a changed polypeptide profile as well as incorporating the most label; one of the polypeptides, Mr 150 000\p=n-\250000, was undetectable, but a train of Mr 70 000\p=n-\90000 polypeptides and a discrete polypeptide at Mr 20 000 were new. Since this changed profile did not occur in zonae from superovulated eggs, or in zonae from eggs fertilized in vitro, a synergism between oviducal factors and factors from the spermatozoon or egg, or both, towards the zona in vivo is indicated. -

Sperm Capacitation in Surgicallyinseminated Gilts
Regional influences of the Fallopian tubes on the rate of boar sperm capacitation in surgically inseminated gilts R. H. F. Hunter, W. T. Huang and W. Holtz Institute ofAnimal Physiology and Genetics, University of Göttingen, Albrecht-Thaer-Weg 3, D-37075 Göttingen, Germany Aliquots of ejaculated boar semen containing known numbers of spermatozoa were deposited into the caudal isthmus or rostral ampulla of the Fallopian tubes of gilts at, or immediately after, ovulation to assess regional influences on the rate of capacitation. Eggs were recovered during a second intervention 4, 5, 6 or 7 h after surgical insemination and were examined by phase-contrast microscopy. Results were obtained from ten animals in each of the 4-, 5- and 6-h groups and from eight animals in the 7-h group. With two exceptions, fertilized eggs were not recovered until 6 h after insemination into the isthmus, the proportion (45.6%) being significantly greater than the corresponding figure (1.4%) for ampullary insemination (P < 0.001). Similarly, the proportion of fertilized eggs recovered 7 h after insemination into the isthmus (58.7%) was significantly greater than after ampullary insemination (21.9%; P < 0.01). Numbers of spermatozoa associated with the zona pellucida remained low in all these instances, with mean figures per egg ranging from 0.3 to 3.8. Insemination into the isthmus gave a 1\p=n-\2h advantage in fertilization compared with insemination into the ampulla. Although relative rates of sperm cell progression to the site of fertilization may have contributed to this, there is strong evidence that rates of capacitation differ significantly in the respective portions of the Fallopian tube. -

A Phylogeographic Survey of the Pygmy Mouse Mus Minutoides in South Africa: Taxonomic and Karyotypic Inference from Cytochrome B Sequences of Museum Specimens
A Phylogeographic Survey of the Pygmy Mouse Mus minutoides in South Africa: Taxonomic and Karyotypic Inference from Cytochrome b Sequences of Museum Specimens Pascale Chevret1*, Terence J. Robinson2, Julie Perez3, Fre´de´ric Veyrunes3, Janice Britton-Davidian3 1 Laboratoire de Biome´trie et Biologie Evolutive, UMR CNRS 5558, Universite´ Lyon 1, Villeurbanne, France, 2 Evolutionary Genomics Group, Department of Botany and Zoology, University of Stellenbosch, Stellenbosch, South Africa, 3 Institut des Sciences de l’Evolution de Montpellier, UMR CNRS 5554, Universite´ Montpellier 2, Montpellier, France Abstract The African pygmy mice (Mus, subgenus Nannomys) are a group of small-sized rodents that occur widely throughout sub- Saharan Africa. Chromosomal diversity within this group is extensive and numerous studies have shown the karyotype to be a useful taxonomic marker. This is pertinent to Mus minutoides populations in South Africa where two different cytotypes (2n = 34, 2n = 18) and a modification of the sex determination system (due to the presence of a Y chromosome in some females) have been recorded. This chromosomal diversity is mirrored by mitochondrial DNA sequences that unambiguously discriminate among the various pygmy mouse species and, importantly, the different M. minutoides cytotypes. However, the geographic delimitation and taxonomy of pygmy mice populations in South Africa is poorly understood. To address this, tissue samples of M. minutoides were taken and analysed from specimens housed in six South African museum collections. Partial cytochrome b sequences (400 pb) were successfully amplified from 44% of the 154 samples processed. Two species were identified: M. indutus and M. minutoides. The sequences of the M. indutus samples provided two unexpected features: i) nuclear copies of the cytochrome b gene were detected in many specimens, and ii) the range of this species was found to extend considerably further south than is presently understood. -

Supplementary Materials
Supplementary materials Supplementary Table S1: MGNC compound library Ingredien Molecule Caco- Mol ID MW AlogP OB (%) BBB DL FASA- HL t Name Name 2 shengdi MOL012254 campesterol 400.8 7.63 37.58 1.34 0.98 0.7 0.21 20.2 shengdi MOL000519 coniferin 314.4 3.16 31.11 0.42 -0.2 0.3 0.27 74.6 beta- shengdi MOL000359 414.8 8.08 36.91 1.32 0.99 0.8 0.23 20.2 sitosterol pachymic shengdi MOL000289 528.9 6.54 33.63 0.1 -0.6 0.8 0 9.27 acid Poricoic acid shengdi MOL000291 484.7 5.64 30.52 -0.08 -0.9 0.8 0 8.67 B Chrysanthem shengdi MOL004492 585 8.24 38.72 0.51 -1 0.6 0.3 17.5 axanthin 20- shengdi MOL011455 Hexadecano 418.6 1.91 32.7 -0.24 -0.4 0.7 0.29 104 ylingenol huanglian MOL001454 berberine 336.4 3.45 36.86 1.24 0.57 0.8 0.19 6.57 huanglian MOL013352 Obacunone 454.6 2.68 43.29 0.01 -0.4 0.8 0.31 -13 huanglian MOL002894 berberrubine 322.4 3.2 35.74 1.07 0.17 0.7 0.24 6.46 huanglian MOL002897 epiberberine 336.4 3.45 43.09 1.17 0.4 0.8 0.19 6.1 huanglian MOL002903 (R)-Canadine 339.4 3.4 55.37 1.04 0.57 0.8 0.2 6.41 huanglian MOL002904 Berlambine 351.4 2.49 36.68 0.97 0.17 0.8 0.28 7.33 Corchorosid huanglian MOL002907 404.6 1.34 105 -0.91 -1.3 0.8 0.29 6.68 e A_qt Magnogrand huanglian MOL000622 266.4 1.18 63.71 0.02 -0.2 0.2 0.3 3.17 iolide huanglian MOL000762 Palmidin A 510.5 4.52 35.36 -0.38 -1.5 0.7 0.39 33.2 huanglian MOL000785 palmatine 352.4 3.65 64.6 1.33 0.37 0.7 0.13 2.25 huanglian MOL000098 quercetin 302.3 1.5 46.43 0.05 -0.8 0.3 0.38 14.4 huanglian MOL001458 coptisine 320.3 3.25 30.67 1.21 0.32 0.9 0.26 9.33 huanglian MOL002668 Worenine -

Mammals of the Kafa Biosphere Reserve Holger Meinig, Dr Meheretu Yonas, Ondřej Mikula, Mengistu Wale and Abiyu Tadele
NABU’s Follow-up BiodiversityAssessmentBiosphereEthiopia Reserve, Follow-up NABU’s Kafa the at NABU’s Follow-up Biodiversity Assessment at the Kafa Biosphere Reserve, Ethiopia Small- and medium-sized mammals of the Kafa Biosphere Reserve Holger Meinig, Dr Meheretu Yonas, Ondřej Mikula, Mengistu Wale and Abiyu Tadele Table of Contents Small- and medium-sized mammals of the Kafa Biosphere Reserve 130 1. Introduction 132 2. Materials and methods 133 2.1 Study area 133 2.2 Sampling methods 133 2.3 Data analysis 133 3. Results and discussion 134 3.1 Soricomorpha 134 3.2 Rodentia 134 3.3 Records of mammal species other than Soricomorpha or Rodentia 140 4. Evaluation of survey results 143 5. Conclusions and recommendations for conservation and monitoring 143 6. Acknowledgements 143 7. References 144 8. Annex 147 8.1 Tables 147 8.2 Photos 152 NABU’s Follow-up Biodiversity Assessment at the Kafa Biosphere Reserve, Ethiopia Small- and medium-sized mammals of the Kafa Biosphere Reserve Holger Meinig, Dr Meheretu Yonas, Ondřej Mikula, Mengistu Wale and Abiyu Tadele 130 SMALL AND MEDIUM-SIZED MAMMALS Highlights ´ Eight species of rodents and one species of Soricomorpha were found. ´ Five of the rodent species (Tachyoryctes sp.3 sensu (Sumbera et al., 2018)), Lophuromys chrysopus and L. brunneus, Mus (Nannomys) mahomet and Desmomys harringtoni) are Ethiopian endemics. ´ The Ethiopian White-footed Mouse (Stenocephalemys albipes) is nearly endemic; it also occurs in Eritrea. ´ Together with the Ethiopian Vlei Rat (Otomys fortior) and the African Marsh Rat (Dasymys griseifrons) that were collected only during the 2014 survey, seven endemic rodent species are known to occur in the Kafa region, which supports 12% of the known endemic species of the country. -

Xenopus Laevis Sperm Receptor Gp69/64 Glycoprotein Is a Homolog
Proc. Natl. Acad. Sci. USA Vol. 96, pp. 829–834, February 1999 Biochemistry Xenopus laevis sperm receptor gp69y64 glycoprotein is a homolog of the mammalian sperm receptor ZP2 JINGDONG TIAN*, HUI GONG, AND WILLIAM J. LENNARZ† Department of Biochemistry and Cell Biology and Institute for Cell and Developmental Biology, State University of New York, Stony Brook, NY 11794-5215 Contributed by William J. Lennarz, November 4, 1998 ABSTRACT Little is known about sperm-binding pro- Much less information is available on the identity of sperm teins in the egg envelope of nonmammalian vertebrate species. receptors in other, nonmammalian vertebrate species. Re- We report here the molecular cloning and characterization of cently, a Xenopus laevis sperm receptor (gp69y64) in the egg a recently identified sperm receptor (gp69y64) in the Xenopus vitelline envelope (VE) was identified (7, 8). It was shown that laevis egg vitelline envelope. Our data indicate that the gp69 the purified gp69y64 proteins, as well as their antibodies, and gp64 glycoproteins are two glycoforms of the receptor and blocked sperm binding to unfertilized eggs or to beads coupled have the same number of N-linked oligosaccharide chains but with gp69y64 proteins. It has been know for some time (9) that differ in the extent of O-glycosylation. The amino acid se- during fertilization, gp69y64 undergoes limited proteolysis. quence of the receptor is closely related to that of the mouse However, the molecular details of this cleavage have been zona pellucida protein ZP2. Most of the sequence conserva- unclear because the primary structure of gp69y64 was un- tion, including a ZP domain, a potential furin cleavage site, known.