Tendon Tissue Engineering: Progress, Challenges, and Translation to the Clinic
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
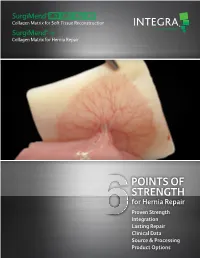
Points of Strength
-e Collagen Matrix for Hernia Repair POINTS OF STRENGTH for Hernia Repair Proven Strength Integration Lasting Repair Clinical Data Source & Processing Product Options IMPORTANCE OF STRENGTH SurgiMend® is offered in thickness of 1mm, 2mm, 3mm and 4mm. Even at 1mm thickness, SurgiMend has been shown to have the strongest uniaxial tensile strength when compared 1 to other major competitors. 30 25 20 15 10 5 Uniaxial Tensile Stress (Mpa) Tensile Uniaxial 0 Surgisis®, SurgiMend Straice™, XenMatrix™ AlloMax™ X-Thick FlexHD® Veritas® Peri-Guard® CollaMend™ CollaMend Permacol™ Biodesign® Firm AlloDerm® FM An MD Anderson study comparing mechanical properties of ADMs derived from bovine and porcine dermis found 3mm and 4mm thick SurgiMend: • To be as strong as the stainless steel suture2 Tear Resistance Thickness Maximum Load ® ™ • To haveSurgiMend twice the suture retentionStrattice of (mm), SD (N), SD porcine dermal matrix (2mm thick)2 PADM* 1.75 + 0.14 19.66† + 3.90 ** † • To be more than twice as resistant to tearing BADM 2.0 1.85 + 0.27 50.95 + 11.79 as porcine dermal matrix2 BADM 3.0 3.23 + 0.54 86.89† + 15.34 BADM 4.0 3.83 + 0.32 100.02† + 14.28 * PADM: Porcine Acellular Dermal Matrix ** BADM: Bovine Acellular Dermal Matrix † Significant difference from all other conditions by 1-way analysis of variance and Tukey post hoc analysis (P < 0.05). • SurgiMend’s increased resistance to tearing is papillary dermis related to the bovine dermal source material2,3 • Bovine and porcine dermis have significant 3 reticular dermis differences in collagen fiber weave pattern • Unlike bovine dermis, hair follicles perforate hypodermis 4 Bovine Porcine the entire dermis in porcine skin INTEGRATION & REVASCULARIZATION SurgiMend is rapidly revascularized to support tissue building and healing for prolonged reinforcement.5,6 11 ½ Months: Revascularized SurgiMend 11 ½ months after hernia repair in a patient undergoing a subsequent procedure unrelated to the original hernia repair. -
Surgimend 1.0 2.0 3.0 4.0 Collagen Matrix for Soft Tissue Reconstruction
® SurgiMend 1.0 2.0 3.0 4.0 Collagen Matrix for Soft Tissue Reconstruction Bioactive, non-inflammatory, rBioactive, tive collagen non-inflammatory, matrix regenerative collagen matrix Rapidly revasculariz Rapidly revascularized and incorporated ted Product Overview SurgiMend is a unique acellular collagen matrix derived from fetal and SurgiMend for soft tissue neonatal bovine dermis. SurgiMend oers clear advantages over synthetic repair, reinforcement & and other biologic products for soft tissue repair and reconstruction. reconstruction. SurgiMend is the Strongest, Thickest Biologic Matrix • Plastic & reconstructive ● The biological make-up of bovine dermis, including its inherent surgery collagen ber architecture, leaves SurgiMend unmatched in available thicknesses and mechanical strength • Muscle flap reinforcement ● 1.0, 2.0, 3.0 & 4.0 mm thicknesses and sizes up to 25 cm x 40 cm • Hernia repair SurgiMend Offers the Highest Levels of Type III Healing Collagen ● Type III collagen mediates tissue healing while inhibiting scarring ● SurgiMend is derived from young healthy tissue that contains three The preferred choice of: times more Type III collagen than other acellular dermal matrices • General Surgeons SurgiMend is Terminally Sterilized, Safe, and Consistent • Trauma Surgeons ● Free of potentially antigenic antibiotics and terminally sterilized ● Derived from only well-dened and characterized source tissue with • Plastic & Reconstructive respect to age, mechanical strength, structure, and composition Surgeons SurgiMend is Non-Inflammatory -

A Collagen and Silk Scaffold for Improved Healing of the Tendon
LAB/IN VITRO RESEARCH e-ISSN 1643-3750 © Med Sci Monit, 2019; 25: 269-278 DOI: 10.12659/MSM.912038 Received: 2018.07.10 Accepted: 2018.09.25 A Collagen and Silk Scaffold for Improved Published: 2019.01.09 Healing of the Tendon and Bone Interface in a Rabbit Model Authors’ Contribution: ACDEF 1 Shengjun Qian 1 Centre for Orthopedic Research, Orthopedics Research Institute of Zhejiang Study Design A BCEF 1 Zhan Wang University, Department of Orthopedics, The Second Affiliated Hospital, School of Data Collection B Medicine, Zhejiang University, Hangzhou, Zhejiang, P.R. China Statistical Analysis C ABDF 1 Zefeng Zheng 2 Department of Orthopedics, Suichang Peoples’ Hospital, Lishui, Zhejiang, Data Interpretation D BCD 1 Jisheng Ran P.R. China Manuscript Preparation E BDF 2 Junfeng Zhu Literature Search F Funds Collection G ACDG 1 Weishan Chen Corresponding Author: Weishan Chen, e-mail: [email protected] Source of support: This work was supported by the Zhejiang Provincial Natural Science Foundation (Grant no. LQ17H060003) Background: The study aimed to develop a novel orthopedic surgical scaffold made of collagen and silk to repair the tendon and bone interface, and to investigate its influence on tendon and bone healing in a rabbit model. Material/Methods: Four types of surgical scaffold were prepared, including a random collagen scaffold (RCS), an aligned collagen scaffold (ACS), a random collagen scaffold combined with knitted silk (RCSS), and an aligned collagen scaffold combined with knitted silk (ACSS). Rabbit bone marrow stem cells (BMSCs) were cultured and seeded onto the RCS and ACS scaffold. The animal model included four-month-old female New Zealand White rabbits (N=20) that underwent drilling into the rotator cuff of the left supraspinatus muscle tendon, randomized into the ACSS and RCSS groups. -

Application of Purified Porcine Collagen in Patients with Chronic
Korean J Pain 2020;33(4):395-399 https://doi.org/10.3344/kjp.2020.33.4.395 pISSN 2005-9159 eISSN 2093-0569 Clinical Research Article Application of purified porcine collagen in patients with chronic refractory musculoskeletal pain Hyunyoung Seong, Raing Kyu Kim, Youngjae Shin, Hye Won Lee, and Jae Chul Koh Department of Anesthesiology and Pain Medicine, Korea University Anam Hospital, Seoul, Korea Received April 27, 2020 Revised August 7, 2020 Background: This study aimed to assess the potential efficacy of purified porcine Accepted August 17, 2020 atelocollagen (PAC) for the management of refractory chronic pain due to suspected connective tissue damage. Handling Editor: Joon-Ho Lee Methods: Patients treated with PAC were retrospectively evaluated. Patients with chronic refractory pain, suspected to have originated from musculoskeletal damage Correspondence or defects with the evidence of imaging studies were included. Pain intensity, using Jae Chul Koh the 11-point numerical rating scale (NRS), was assessed before the procedure, and Department of Anesthesiology and 1 month after the last procedure. Pain Medicine, Korea University Anam Results: Eighty-eight patients were finally included for investigation. The mean NRS Hospital, 73 Goryeodae-ro, Seongbuk-gu, Seoul 02841, Korea score was decreased from 5.8 to 4.1 after 1 month of PAC injection (P < 0.001). No Tel: +82-2-920-5632 independent factor was reported to be directly related to the decrease in NRS score Fax: +82-2-920-5630 by more than half. E-mail: [email protected] Conclusions: Application of PAC may have potential as a treatment option for re- fractory chronic musculoskeletal pain. -

No.7 July 2004
Vol.47, No.7 July 2004 CONTENTS JMA Policies Policy Address Haruo UEMATSU . 305 Regenerative Medicine Regenerative Medicine for Cartilage Defects Mitsuo OCHI . 307 The Use of Skin Regeneration Technique in the Treatment of Full-Thickness Skin Defects Norio KUMAGAI . 311 Regenerative Medicine for the Cornea Shigeru KINOSHITA and Takahiro NAKAMURA . 317 Regenerative Medicine for Sclerotic Disorders Toshikazu NAKAMURA . 322 Regenerative Medicine for Cardiomyocytes Keiichi FUKUDA . 328 Regenerative Medicine for Respiratory Diseases Tatsuo NAKAMURA . 333 Centenarians Implications of Research Findings Obtained from Centenarians Hiroshi SHIBATA . 338 Abdominal Aortic Aneurysm Forefront of Treatment for Abdominal Aortic Aneurysm Hiroshi YASUHARA . 344 ⅥJMA Policies Policy Address JMAJ 47(7): 305–306, 2004 Haruo UEMATSU President, Japan Medical Association The 110th General Assembly of the JMA House of Delegates was held for two days from April 1 to 2 at the JMA Hall. Elections for the offices of the executive board were held on the first day and Dr. Haruo Uematsu (former president of the Osaka Medical Association) was elected as the 16th president of the JMA for the first time. The follow- ing is a main part of the policy address of Dr. Uematsu presented on April 2, 2004. Our country is facing a period of great tional women, to foster their capabilities, and changes as can be seen in the dispatch of Self- to send them to the JMA in future. Defense Forces to Iraq by the government of Since this is such an important time for the Japan amidst an ongoing debate about revising JMA, I believe that making the organization’s the Japanese Constitution. -

Qt9661q7w2.Pdf
UCLA UCLA Previously Published Works Title The Role of the Anti-Inflammatory Cytokine Interleukin-10 in Tissue Fibrosis. Permalink https://escholarship.org/uc/item/9661q7w2 Journal Advances in wound care, 9(4) ISSN 2162-1918 Authors Steen, Emily H Wang, Xinyi Balaji, Swathi et al. Publication Date 2020-04-01 DOI 10.1089/wound.2019.1032 Peer reviewed eScholarship.org Powered by the California Digital Library University of California COMPREHENSIVE INVITED REVIEW The Role of the Anti-Inflammatory Cytokine Interleukin-10 in Tissue Fibrosis Emily H. Steen,1,2 Xinyi Wang,2 Swathi Balaji,2 Manish J. Butte,3 Paul L. Bollyky,4 and Sundeep G. Keswani1,2,5,* 1Department of Surgery, Baylor College of Medicine, Houston, Texas. 2Laboratory for Regenerative Tissue Repair, Texas Children’s Hospital, Houston, Texas. 3Division of Immunology, Allergy, and Rheumatology, Department of Pediatrics, University of California, Los Angeles, Los Angeles, California. 4Division of Infectious Diseases, Department of Medicine, Stanford University School of Medicine, Stanford, California. 5Division of Pediatric Surgery, Department of Surgery, Texas Children’s Hospital, Houston, Texas. Significance: Fibrosis is the endpoint of chronic disease in multiple organs, including the skin, heart, lungs, intestine, liver, and kidneys. Pathologic accu- mulation of fibrotic tissue results in a loss of structural integrity and function, with resultant increases in morbidity and mortality. Understanding the path- ways governing fibrosis and identifying therapeutic targets within those path- ways is necessary to develop novel antifibrotic therapies for fibrotic disease. Recent Advances: Given the connection between inflammation and fibrogenesis, Interleukin-10 (IL-10) has been a focus of potential antifibrotic therapies because of its well-known role as an anti-inflammatory mediator. -

Tendon Proper- and Peritenon-Derived Progenitor Cells Have Unique Tenogenic Properties Michael J Mienaltowski1,2,3*, Sheila M Adams1 and David E Birk1,2
Mienaltowski et al. Stem Cell Research & Therapy 2014, 5:86 http://stemcellres.com/content/5/4/86 RESEARCH Open Access Tendon proper- and peritenon-derived progenitor cells have unique tenogenic properties Michael J Mienaltowski1,2,3*, Sheila M Adams1 and David E Birk1,2 Abstract Introduction: Multipotent progenitor populations exist within the tendon proper and peritenon of the Achilles tendon. Progenitor populations derived from the tendon proper and peritenon are enriched with distinct cell types that are distinguished by expression of markers of tendon and vascular or pericyte origins, respectively. The objective of this study was to discern the unique tenogenic properties of tendon proper- and peritenon-derived progenitors within an in vitro model. We hypothesized that progenitors from each region contribute differently to tendon formation; thus, when incorporated into a regenerative model, progenitors from each region will respond uniquely. Moreover, we hypothesized that cell populations like progenitors were capable of stimulating tenogenic differentiation, so we generated conditioned media from these cell types to analyze their stimulatory potentials. Methods: Isolated progenitors were seeded within fibrinogen/thrombin gel-based constructs with or without supplementation with recombinant growth/differentiation factor-5 (GDF5). Early and late in culture, gene expression of differentiation markers and matrix assembly genes was analyzed. Tendon construct ultrastructure was also compared after 45 days. Moreover, conditioned media from tendon proper-derived progenitors, peritenon-derived progenitors, or tenocytes was applied to each of the three cell types to determine paracrine stimulatory effects of the factors secreted from each of the respective cell types. Results: The cell orientation, extracellular domain and fibril organization of constructs were comparable to embryonic tendon. -

6174.Full-Text.Pdf
Monocyte Chemoattractant Protein-1 Enhances Gene Expression and Synthesis of Matrix Metalloproteinase-1 in Human Fibroblasts by an Autocrine IL-1 α Loop This information is current as of September 30, 2021. Toshiyuki Yamamoto, Beate Eckes, Cornelia Mauch, Karin Hartmann and Thomas Krieg J Immunol 2000; 164:6174-6179; ; doi: 10.4049/jimmunol.164.12.6174 http://www.jimmunol.org/content/164/12/6174 Downloaded from References This article cites 39 articles, 18 of which you can access for free at: http://www.jimmunol.org/content/164/12/6174.full#ref-list-1 http://www.jimmunol.org/ Why The JI? Submit online. • Rapid Reviews! 30 days* from submission to initial decision • No Triage! Every submission reviewed by practicing scientists • Fast Publication! 4 weeks from acceptance to publication by guest on September 30, 2021 *average Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2000 by The American Association of Immunologists All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. Monocyte Chemoattractant Protein-1 Enhances Gene Expression and Synthesis of Matrix Metalloproteinase-1 in Human Fibroblasts by an Autocrine IL-1␣ Loop1 Toshiyuki Yamamoto,2 Beate Eckes, Cornelia Mauch, Karin Hartmann, and Thomas Krieg Monocyte chemoattractant protein-1 (MCP-1), a member of the C-C chemokine superfamily, has recently been shown to be involved in the pathogenesis of tissue fibrosis. -

Surgimend® 1.0 2.0 3.0 4.0 Collagen Matrix for Soft Tissue Reconstruction
Product Summary ® SurgiMend 1.0 2.0 3.0 4.0 Collagen Matrix for Soft Tissue Reconstruction Because we are committed to limiting uncertainty, Integra offers SurgiMend in 1, 2, 3 or 4 mm thicknesses and in over sixty configurations, providing the most appropriate device thickness, strength, and size for each procedure, technique, and patient. SurgiMend® 1.0 2.0 3.0 4.0 Collagen Matrix for Soft Tissue Reconstruction Product Overview SurgiMend® is a unique acellular collagen matrix derived from fetal and neonatal bovine dermis. SurgiMend offers clear advantages over synthetic and other biologic products for soft tissue repair and reconstruction. SurgiMend is the Strongest, Thickest Biologic Matrix 9 • The biological make-up of bovine dermis, including its inherent collagen fiber architecture, leaves SurgiMend unmatched in available thicknesses and mechanical strength • 1.0, 2.0, 3.0 and 4.0 mm thicknesses and sizes up to 25 cm x 40 cm SurgiMend Offers an Abundance of Type III Healing Collagen 3,4 • Type III collagen mediates tissue healing while inhibiting scarring • SurgiMend is derived from young healthy tissue that contains three times more Type III collagen than other acellular dermal matrices SurgiMend is Terminally Sterilized, Safe, and Consistent • Free of potentially antigenic antibiotics and terminally sterilized • Derived from only well-defined and characterized source tissue with respect to age, mechanical strength, structure, and composition SurgiMend is Non-Inflammatory for Better Reinforcement • Does not elicit an acute or chronic -

In Vitro Differentiation of Adipose-Derived Mesenchymal Stem Cell Into Insulin-Producing Cells
OPEN ACCESS http://scidoc.org/IJST.php International Journal of Stem Cell Research and Transplantation (IJST) ISSN: 2328-3548 In vitro Differentiation of Adipose-Derived Mesenchymal Stem Cell into Insulin-Producing Cells Research Article Babiker NE1, Gassoum A2, Musa HH1, Hamed ALDeaf SA2, Abdelraheem NE2,3, Fadl-Elmula I6, Ali El-Sheikh MA4, Abdelrahman Arbab M2,5* 1 Faculty of Medical Laboratory Sciences, University of Khartoum, Khartoum, Sudan. 2 National Center of Neurological Sciences, Khartoum, Sudan. 3 Faculty of Medical Laboratory Sciences, National University, Khartoum, Sudan. 4 Faculty of Medicine, University of Khartoum, Khartoum, Sudan. 5 Faculty of Medicine, Department of Surgery, University of Khartoum, Khartoum, Sudan. 6 Faculty of Medicine, Al Neelain University, Khartoum, Sudan. Abstract Adipose or fatty tissue is similar to bone marrow ontogenetically. Mesenchymal stem cells can be isolated from different types of adipose tissue depots in greater amount than other sources, making them especially suitable for use in regenerative medicine. Adipose tissue was taken from Sudanese donors; the SVF which contains MSCs was isolated using enzymatic and density centrifugation techniques. The AD-MSCs were differentiated to insulin producing cells using three steps protocol. The therapeutic effect of islet β-like cells was determined in vivo using diabetics albino Wister rats. The adherent cells firstly appeared round and spherical in shape, the characteristic shape of MSC was detected after three weeks incubation. The pheno type of these cells showed positives CD34 and CD13 and negatives CD45 and HLADR markers. AD-MSCs were induced into insulin producing cells by a 3-step (15-days) protocol. The differentiated cells were positive for diathizone stain and displayed positive immuno-reactivity to antihuman insulin antibody. -

UNIVERSITY of WISCONSIN-LA CROSSE Graduate Studies
UNIVERSITY OF WISCONSIN-LA CROSSE Graduate Studies INCREASE OF EXTRACELLULAR MATRIX PROTEINS IN HIBERNATING GROUND SQUIRRELS COULD HELP MAINTAIN BONE HEALTH A Manuscript Style Thesis Submitted in Partial Fulfillment of the Requirements for the Degree of Master of Science – Biology: Physiology Concentration Hannah Bergen College of Science and Health Biology: Physiology Concentration December, 2018 ABSTRACT Bergen, H.R. Increase of extracellular matrix proteins in hibernating ground squirrels could help maintain bone health. MS in Physiology, December 2018, pp.43. (S. Cooper) Whether from injury, old age, or space flight, immobility or a lack of gravitational loading has negative effects on the physiology of bone and bone marrow. Consequences include a weakening of the immune system, decreased hematopoietic cells in the bone marrow, and an overall decrease in bone density. Understanding the mechanisms behind these effects and combating their side effects is a subject of intense research. Thirteen lined ground squirrels are a common model organism for these studies because they hibernate for months and experience long periods of inactivity. Like humans under low loading and activity, the ground squirrels experience changes in the bone marrow cell make up and decreased bone density, but, unlike humans, once a 13-lined ground squirrel becomes active again, the side effects from their long rest are minute or non-existent. Transcriptomes were constructed from genes expressed in bone marrow from hibernating and non-hibernating ground squirrels. Certain extracellular matrix proteins, including COL4A2, were seen to increase drastically during hibernation and may possibly play a role in maintaining the health of the bone. iii ACKNOWLEDGEMENTS I want to thank my dad and mom, for shaping me into who I am today, for believing in me and supporting me, not just through school but each step and decision life presents. -

Trix Consisting of Citric Acid and Collagen
Advance Online Publication © Annals of Vascular Diseases 2011 doi:10.3400/avd.oa.11.00017 Original Article Enhanced Neovascular Formation in a Novel Hydrogel Ma- trix Consisting of Citric Acid and Collagen Mikiko Nagayoshi, MD,1, 2 Tetsushi Taguchi, PhD,3 Hiroyuki Koyama, MD,1, 2 Tsuyoshi Takato, MD,2 Tetsuro Miyata, MD,1 and Hirokazu Nagawa, MD1 Background: Three-dimensional regenerative tissue with large bulk generally requires blood perfusion through a vascular network to maintain its viability, and one promising approach is induction of neovascu- lar growth from the recipient bed into the tissue. To induce ingrowth of a vascular network, it is necessary to furnish the regenerative tissue with a scaffold structure for neovasculature and a delivery system for an angiogenic growth factor. As such a scaffold structure, the present study created novel hydrogel materials by chemically cross-linking alkali-treated collagen (AlCol) with trisuccinimidyl citrate (TSC). Materials and Methods: Many prototypes, consisting of several concentrations of TSC and AlCol, were implanted into the subfascial space of the rat rectus muscle, and 7 days later, the implanted materials were excised for histological analysis. Cross-sections were stained and neovascular development in the materials was evaluated by measuring vessel density, length and number of joints and branches. Results: Significant ingrowth of vascularized granulation was observed in some materials, which surpassed the angiogenic ability of MatrigelTM. Further, combination with basic fibroblast growth factor (bFGF) sig- nificantly increased the vascular formation in these gels. Conclusions: The TSC-AlCol gel functioned as a favorable scaffold for neovascular formation and also as a reservoir for controlled delivery of bFGF.