Official Journal L101
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Corporate Social Responsibility and Sustainability Report 2016
Public Power Corporation S.A. 30 Halkokondyli St., Athens GR-10432, Τel.: +30 210 523 0301 www.dei.gr CONTENTS CONTENTS 1. MESSAGE FROM THE CHAIRMAN AND CEO 6 2. ABOUT THE REPORT 10 3. PPC CORPORATE PROFILE 14 3.1. ACTIVITIES 14 3.2 SHAREHOLDER STRUCTURE 20 3.3 HOLDINGS IN SUBSIDIARIES 20 3.4 CORPORATE GOVERNANCE FRAMEWORK 21 3.5 ADMINISTRATIVE ORGANISATION 21 3.6 GOVERNANCE STRUCTURE 23 3.7 CONFLICT OF INTEREST 26 3.8 AUDITS 26 3.9 RISK AND CRISIS MANAGEMENT 27 3.10 ENERGY MARKET OPERATIONS AND PUBLIC POLICY 29 3.11 NEW MARKETS AND INVESTMENTS 32 3.12 KEY FINANCIAL INFORMATION 33 4. SUSTAINABLE DEVELOPMENT 36 4.1 MANAGEMENT APPROACH 36 4.2 GOVERNANCE FOR SUSTAINABLE DEVELOPMENT ISSUES 41 4.3 MATERIALITY ANALYSIS 42 4.4 STAKEHOLDERS 48 4.5 MEMBERSHIP OF ASSOCIATIONS AND ORGANISATIONS 53 4.6 AWARDS - DISTINCTIONS 53 4.7 KEY CORPORATE SOCIAL RESPONSIBILITY PERFORMANCE DATA 54 4.8 COMMITMENTS - GOALS 56 5. EMPLOYEES 60 5.1 HUMAN RESOURCES DATA 60 5.2 TRAINING AND DEVELOPMENT 67 5.3 EMPLOYEE EVALUATION AND BENEFITS 69 5.4 EQUAL OPPORTUNITIES AND RESPECT FOR HUMAN RIGHTS 70 5.5 HEALTH & SAFETY 70 5.6 INTERNAL COMMUNICATION 78 5.7 REGULATORY AND LEGISLATIVE COMPLIANCE 79 4 CORPORATE SOCIAL RESPONSIBILITY AND SUSTAINABILITY REPORT 2016 6. ENVIRONMENT 80 6.1 ENVIRONMENTAL MANAGEMENT 80 6.2 CONSUMPTION OF RAW MATERIALS, FUELS AND ENERGY 83 6.3 GREENHOUSE GAS AND OTHER GAS EMISSIONS 85 6.4 ACTIONS TO REDUCE GREENHOUSE GAS EMISSIONS 92 6.5 WATER MANAGEMENT 95 6.6 WASTE MANAGEMENT - USE OF BY-PRODUCTS 100 6.7 BIODIVERSITY 105 6.8 REGULATORY AND LEGISLATIVE COMPLIANCE 109 7. -

Horaires Et Trajet De La Ligne 344 De Bus Sur Une Carte
Horaires et plan de la ligne 344 de bus 344 Boulogne sur Gesse - Saint Gaudens Voir En Format Web La ligne 344 de bus (Boulogne sur Gesse - Saint Gaudens) a 2 itinéraires. Pour les jours de la semaine, les heures de service sont: (1) Boulogne-Sur-Gesse / Saint-Gaudens: 07:00 - 13:52 (2) Saint-Gaudens / Boulogne-Sur-Gesse: 13:06 - 18:44 Utilisez l'application Moovit pour trouver la station de la ligne 344 de bus la plus proche et savoir quand la prochaine ligne 344 de bus arrive. Direction: Boulogne-Sur-Gesse / Saint-Gaudens Horaires de la ligne 344 de bus 19 arrêts Horaires de l'Itinéraire Boulogne-Sur-Gesse / Saint- VOIR LES HORAIRES DE LA LIGNE Gaudens: lundi 07:00 - 13:52 Boulogne-Sur-Gesse - Collège mardi 07:00 - 13:52 Boulogne-Sur-Gesse - Hlm mercredi 07:00 - 13:52 Rue de l'Aneto, Boulogne-sur-Gesse jeudi 07:00 - 13:52 Blajan - Mairie vendredi 07:00 - 13:52 Blajan - Cité Bellevue samedi 13:52 Blajan - La Tuilerie dimanche Pas opérationnel Saint-Pe-Delbosc - Rebirechioulet Saint-Pe-Delbosc - Rebirechioulet Informations de la ligne 344 de bus Ciadoux - Centre Direction: Boulogne-Sur-Gesse / Saint-Gaudens Arrêts: 19 Durée du Trajet: 11 min Montgaillard-Sur-Save - Cimetière Récapitulatif de la ligne: Boulogne-Sur-Gesse - Collège, Boulogne-Sur-Gesse - Hlm, Blajan - Mairie, Saman - Calvaire Blajan - Cité Bellevue, Blajan - La Tuilerie, Saint-Pe- Delbosc - Rebirechioulet, Saint-Pe-Delbosc - Saint-Lary-Boujean - Saint-Patatin Rebirechioulet, Ciadoux - Centre, Montgaillard-Sur- Save - Cimetière, Saman - Calvaire, Saint-Lary- Saint-Marcet -

Trie-Sur-Baïse Castelnau Magnoac
h,ϰϯŬŵ &(175(9,//( 9( 8 1( ( / 85 ,/ 9 72 &+(0,1 $ '( ( (/ 58 ' (' (/$& 7285 6 $ 58 / ( 35 5 52 20 5 ( $ , , 5,66( 1 ' D ; $' /E ( ( ' '( 58( d /$ ' ZKh> 6 ( ( 7, ,75 '¶(63$*1( 3 & / 3 + /( +$ 6 8 & & , / 8 (5 6 ' ( 6$//('(6 3 5( 0( 0 86 )Ç7(6 , 3/ 2 $ 9 / 2 ' 28 ('( / 7 ( 3/$& $ ( (6' /,6( /¶(* ,/ / '( ' 8& ( &' 3/$&('( /( $ ( 58 7(/ /¶ (67(//(77( 58 (6 (6 58('8&)3 5 5 8 58 8 3 5 ( ( , 5% ( ' 0 (69& $ 5 55 $ ,03'( $ 8% * ( 7 ( ( 81,6 /$*$5'( ( 8 '( 6 & (7 2 ,/ '( 7 +( $5 6 / $ * 0 7 6 %$ 5 (' $ ,, $ ,( 57 , / ('( 1 3( + 3/$&( , +$ '5 0 6& 1 2 '(/ 58 58 $ 1 ( '( ' 5 3$8/628/(6 3/$&( ( * 5 '( 58 /$3285&$28 8 ' 58 ( ( ( '8 / & / $/ 58 $ ' ( ¶2 ' 66 8 $7 * 7 e1 e5$ ,( 85 / $% 58( (& '¶$5 '(6( (<=(77( &2/( 6 */ '(5 78&28/(7,0 /$ ( 3 6 ' ( ( ,0 58 6 1 3' - /((6 ( $5 6+257(1 58('8 * 6, ' $6 , $/ 1 $ 6 7 &+(0,1'( 9 1 8 6,9 +2 '( 6 ( ( ' 58 1 , (0 + & 5287('(/$0$548( 5287('867$'( ^ 'Z h Z/ ^ ZKhd E / D , Z K h h d / h ,D/E^ dͲ > WZsE,^ ZKhdWZ ,D/E h,ϰϯŬŵ KZEhs ,D/E^ E ZK^^dZD/Z^ >/' > dZ/ͳ^hZͳ4^ϭϱŬŵ E dZ^ϰϰŬŵ &(175(9,//( / D YRLUSODQ , E ,D/E^ / /Z GpWDLOOp D >hZ/Z^ > , W d/ ,D/E dKE W K d/d /E d KE D , KE d > > ^ ' W Z/ ͛ D/E > , h >Kd/^^DEd K ZK> h ZK K hd : Zh Zh h h /E 9( E &(175(9,//( ^ KDD/ ,D/ WZ K 8 Yh >^ E' Z ^ /E D D 1( /DW^^> > ,D/E ( d , ZKh / ZZZ^^ 85 ,/ 9 , ^ 72 > ^ W &+(0,1 d $ K '( h,ϰϯŬŵ Z d ( ^ (/ >> 58 ' (' d,ZD^ͳD'EKϴŬŵ (/$& 7285 6 $ , 58 / ( E D/E 5 D 52 35 5 ' 20 Zd/E Kh>K'Eͳ^hZͳ'^^ϭϯŬŵ ( $ , , 5,66( 1 ' D ͛KZ ; $' /E ('( -

Légende Nestalas Uz Soulom Arcizans- Limites Cantonales Avant Villelongue Beaucens Limites Communales
N PLAN LOCAL D’URBANISME Ouzous Salles Agos- COMMUNE DE CAUTERETS Vidalos Ayzac- LOCALISATION Sère-en-Lavedan Ost Boô- Gez Silhen Saint- Pastous ARGELES-Ayros- GAZOST Arbouix Lau-Préchac Balagnas Vier- Saint- Bordes Savin Adast Artalens- Souin Pierrefitte- Légende Nestalas Uz Soulom Arcizans- Limites cantonales Avant Villelongue Beaucens Limites communales Surface communale Cauterets Saint- Lanne Castelnau- Rivière-Basse Madiran Hères Soublecause Labatut- Rivière Hagedet Caussade- Rivière Villefranque Lascazères Estirac Auriébat Sombrun Sauveterre Maubourguet Lahitte- Toupière Vidouze Monfaucon Larreule Lafitole Buzon Gensac Nouilhan Ansost Barbachen Caixon Liac Artagnan Ségalas Vic-en- Villenave- Bigorre Sarriac- Rabastens- près- Sanous Bigorre de-Bigorre Béarn Saint- Mingot Lézer Bazillac Estampures Escaunets Camalès Fréchède Lacassagne (Canton de Vic-en-Bigorre) Sénac Moumoulous Escondeaux Villenave- Saint-Sever- Mazerolles Talazac Pujo près- Ugnouas Marsac de-Rustan Séron Tarasteix Lescurry Mansan Bernadets- Marsac Tostat Bouilh- Debat Siarrouy Peyrun Sarniguet Castéra-Lou Laméac Devant Fontrailles Andrest Antin Lagarde Soréac Trouley- Oroix Gayan Jacque Aurensan Dours Bouilh- Labarthe Lapeyre Trie-sur- Louit Péreuilh Lubret- Gardères Pintac Chis Saint-Luc Lalanne- Baïse Sadournin Guizerix Peyret- Oursbelille Bazet Osmets Trie Collongues Marseillan Saint-André (Canton d'Ossun) Sabalos Luby- Sariac- Orleix Chelle- Betmont Vidou Larroque Casterets Bours Debat Puntous Magnoac Luquet Bordères- Oléac-Debat Castelvieilh Mun Thermes- sur-l'Echez -

ANNEXE 12 Direction Académique De La Haute Garonne ZONES INFRA POUR VOEUX LARGES DPE 5 8 Zones - Par Zone Géographique
Rectorat de Toulouse MOUVEMENT 2021 ANNEXE 12 Direction académique de la Haute Garonne ZONES INFRA POUR VOEUX LARGES DPE 5 8 zones - Par zone géographique CIRCONSCRIPTIONS code ZONE INFRA SECTEURS COMMUNES RATTACHEES 2021 circo 1 - 2 - 3 - 31000 - 31100 - 31200 - 20211 - TOULOUSE TOULOUSE 4 - 5 - TOULOUSE 19 - 24 31300 - 31400 - 31500 Colomiers COLOMIERS AUSSONNE COLOMIERS 17 DAUX Aussonne MERVILLE MONDONVILLE FROUZINS PLAISANCE DU TOUCH 16 TOURNEFEUILLE Frouzins Seysses SEYSSES TOURNEFEUILLE BRAX LA SALVETAT ST GILLES LASSERRE-PRADERE Lévignac LEGUEVIN 20212 - Haute-Garonne LEVIGNAC OUEST MONTAIGUT SUR SAVE PIBRAC BRIGNEMONT BRETX LEGUEVIN 26 CADOURS COX LAUNAC LE BURGAUD Cadours LE CASTERA MENVILLE PELLEPORT ST CEZERT ST PAUL SUR SAVE THIL BEAUZELLE Blagnac BLAGNAC CORNEBARRIEU BLAGNAC 18 FENOUILLET GAGNAC SUR GARONNE Fenouillet LESPINASSE SEILH CEPET GRATENTOUR Gratentour LABASTIDE ST SERNIN ST SAUVEUR VACQUIERS AUCAMVILLE BRUGUIERES BRUGUIERES 6 CASTELGINEST FONBEAUZARD LAUNAGUET Pechbonnieu MONTBERON PECHBONNIEU 20213 - Haute-Garonne ST ALBAN ST GENIES BELLEVUE NORD ST LOUP CAMMAS BOULOC FRONTON Fronton ST RUSTICE VILLENEUVE LES BOULOC BESSIERES BUZET SUR TARN BONDIGOUX BESSIERES LA MAGDELAINE SUR TARN MIREPOIX SUR TARN GRENADE 23 MONTJOIRE CASTELNAU D ESTRETEFONDS GRENADE Ondes LARRA ONDES ST JORY LE BORN VILLAUDRIC VILLEMUR VILLEMATIER VILLEMUR SUR TARN Edition du 23/03/2021 Page 1/6 Rectorat de Toulouse MOUVEMENT 2021 ANNEXE 12 Direction académique de la Haute Garonne ZONES INFRA POUR VOEUX LARGES DPE 5 8 zones - Par zone géographique -

Un 'Tour' Por Los Pirineos
60_63_rutapirineos_MK 23/06/11 12:10 Página 60 VIAJEROS 1 Un ‘tour’ por los Pirineos CADA AÑO EL TOUR SE DESLIZA POR LAS CARRETERAS DE LOS DEPARTAMENTOS DE ARIÈGE, HAUTE GARONNE Y HAUTES PYRÉNNÉES. LOS ESFORZADOS CICLISTAS APENAS PUEDEN SABOREAR EL PAISAJE Y LOS GRANDES PUERTOS SON LOS OBJETIVOS A BATIR. POR JORDI BASTART. ientras los ciclistas apenas levan- Un bello recorrido de 44 km por la D117 1. SUPERBAGNÈRES tan los ojos del asfalto, los auto- acerca al tranquilo pueblecito de Saint Gi- CON UN ENCANTO DISTINTO, EN VERANO, IDEAL PARA M movilistas sí tienen la posibili- rons, capital del valle de Couserans. A ape- RECORRER A PIE O BTT. dad –casi obligación– de parar en los nas un par de kilómetros, es de visita obli- 2. LE CARRÉ DE L’ANGE COCINA TRADICIONAL CON miradores que salpican el recorrido del gada el núcleo de Saint Lizier, lugar de paso TOQUE CREATIVO EN SAINT Tour de Francia, compartiendo ruta con los de una variante del Camino de Santiago, LIZIER. 3. CICLISTAS AFICIONADOS EMULAN A aficionados que emulan a los profesionales. donde los peregrinos acudían a venerar los LAS ESTRELLAS EN EL COL restos del santo. Hoy, el templo dedicado a D’ASPIN. 4. TELEFÉRICO En territorio cátaro san Lizier es una importante muestra de ar- PARA DISFRUTAR DE LAS VISTAS A LO LARGO DE LA El itinerario empieza con la vista puesta en te románico y gótico, con especial atención ASCENSIÓN AL PIC DU MIDI. las tres torres del castillo de Foix, que se yer- a las pinturas murales del siglo XI sobre el guen al paso del río Ariège. -

PRO V· I N C I a DI TORINO
'701 PRO v·I N CI A DI TORINO CIRCONDARIO DI TORINO MISURE LOCALI MISURE METRICHE ~ COMUNI VALORE VALORE DENOMINAZIOì'iE In DENOMINAZIONE in )llSURE METRICHE )J1SURE LOCALI MISURE DI LUNGHEZZA ante1'iormente al 18 t 8 Metri Trabucchi Trabucco ....... ......... 3,082596 Metro 0,324-402 Piedi liprondi Piede liprando •.•••....... 0,54 3766 Id. 1,9464-H Piedi manuali Piede manuale .....•.•.•.. 0,342M t Id. 2,94 9614 TuTTI 1 CoMUNI DEL CIRCONDARIO ....... Piedi legali Piede legale . .•..•.•...... 0,292564 Id. 3,418088 Rasi Raso ........•....••....• 0,599394 Id. 4,66835~ ' Tese . Tesa ...... ~ .....••..••.. 1,712553 Id. 0,583923 poster·iol'mente al 184 8 Metri Trabucchl i Trabucco •••••• •• •• • • ••• o 3,086420 Metro 0,324-000 Piedi liprandi Piede liprando ............ 0,54U03 Id. 4,9UOOO Piedi manuali l Piede manuale ... ....... .. 0,342936 Id. 2,916000 TuTTI I CoMUNI DEL CiRCONDARIO .... Piedi legali Piede legale . ............. 0,292924. Id. 3,413854- Rasi Raso . ....•............ · 0,6004 37 Id. 1,666286 Tese Tesa .•••.••.•••• . 1,714678 Id. 0,583200 Trabucchi CASALBORGONE .•......•••••••..•..... Trabucco antico . o. o ••••• • 3,24.0000 Id. 0,30864.2 l\loNT ANARO. • . • . • . • ....... , Trabucco . .... .. .... .. 3,2304.00 Id. 0,309559 FELETTO .....••...••..•.••.•.. •.... Trabucco abaziale •• : • . ... 3,160000 Id. 0,3164.56 l Il Piede liprando si divide in 12 Once, l'Oncia in 12 Punti, il Punto in 12 Atomi. 6 Piedi formano il Trabucco. 800 Trabucchi formano il Miglio, misura itinerar1a. Il Piede manuale si divide m 8 Oncia, !l l'Oncia in 12 Punti. 5 Piedi manuali, ossia 40 Once formano la Tesa. Il Piede legale corrisponde ad Once 6 e Punti lO del Piede hprando. -

SIAEP ARMAGNAC TENAREZE (Siren : 253200240)
Groupement Mise à jour le 01/07/2021 SIAEP Armagnac-Ténarèze (Siren : 253200240) FICHE SIGNALETIQUE BANATIC Données générales Nature juridique Syndicat mixte fermé Syndicat à la carte oui Commune siège Eauze Arrondissement Condom Département Gers Interdépartemental non Date de création Date de création 02/02/1967 Date d'effet 15/09/2008 Organe délibérant Mode de répartition des sièges Autre cas Nom du président M. Nicolas MELIET Coordonnées du siège Complément d'adresse du siège Mairie Numéro et libellé dans la voie Distribution spéciale Code postal - Ville 32800 EAUZE Téléphone Fax Courriel Site internet Profil financier Mode de financement Aucune contribution budgétaire Bonification de la DGF non Dotation de solidarité communautaire (DSC) non Taxe d'enlèvement des ordures ménagères (TEOM) non Autre taxe non Redevance d'enlèvement des ordures ménagères (REOM) non Autre redevance non Population 1/3 Groupement Mise à jour le 01/07/2021 Population totale regroupée 12 077 Densité moyenne 25,98 Périmètres Nombre total de membres : 16 - Dont 14 communes membres : Dept Commune (N° SIREN) Population 32 Beaumont (213200371) 140 32 Bretagne-d'Armagnac (213200645) 422 32 Castelnau d'Auzan Labarrère (200057586) 1 257 32 Cazeneuve (213201007) 159 32 Eauze (213201197) 4 013 32 Fourcès (213201338) 263 32 Gondrin (213201494) 1 230 32 Lagraulet-du-Gers (213201809) 578 32 Larressingle (213201940) 218 32 Larroque-sur-l'Osse (213201973) 235 32 Lauraët (213202039) 254 32 Montréal (213202906) 1 200 32 Mouchan (213202922) 409 32 Réans (213203409) 293 - Dont -

Carte Planning Déploiement FTTH 2018-2024
Déploiements FTTH ORANGE Période 2018 2024 ± Saint-lanne Castelnau-Riviere-Basse Madiran Heres Soublecause Labatut-riviere Légende Hagedet Caussade-riviere Lascazeres 2018 Villefranque Estirac Auriebat 2019 Sombrun Sauveterre 2020 Maubourguet Lahitte-toupiere 2021 Monfaucon Vidouze Larreule Lafitol e Buzon Gensac 2022 Nouilhan Ansost Barbachen Liac 2023 Caixon Segalas Artagnan 2024 Sarriac-bigorre Vic-En-Bigorre Rabastens-De-Bigorre Sanous Estampures Villenave-pres-bearn Mingot Saint-lezer Frechede Camales Moumoulous Bazillac Lacassagne Senac Mazerolles Pujo Saint-sever Escaunets Ugnouas Bernadets Villenave -de-rustan Escondeaux Mansa -debat Talazac -pres-marsac n Bouilh Lameac Fontrailles Seron Marsac Lescurry Peyrun -devant Antin Siarrouy Tostat Tarasteix Castera-lou Lapeyre Andrest Sarniguet Jacque Trouley Trie-Sur-Baise Guizerix Peyret Soreac Bouilh -labarthe Lubret-saint-luc Aurensan -saint-andre Oroix Gayan -pereu Lalanne-trie Sadournin Lagarde Dours ilh Osmets Sariac Casterets Chis Louit Marseillan Larroque -magnoac Oursbelille Luby Vidou Puntous Garderes Pintac Bazet Chelle-debat -betmont Thermes Collongues Puydarrieux Castelnau Sabalos Castelvieilh Tournous -magnoac Villembits Hachan -Magnoac Bours Oleac Mun -darre Barthe Betbeze Orleix Pouyastruc Cabanac Lamarque Luquet Borderes-Sur-L-Echez -debat Campuzan -rustaing Organ Aries-espenan Lizo Aubarede Lustar Lalanne s Sere Betpouy Deveze Marquerie Sentous Thuy -rustaing Aureilhan Boulin Cizos Pouy Hourc Peyriguere Bugard Vieuzos Coussan Tournous Villemur Goudon Ibos Souyeaux Bonnefont -

Zonages Agricoles Hautes-Pyrénées Classement En Zone Défavorisée Pour Paiement De L'indemnité Compensatoire Carte 1-3 De Handicaps Naturels (ICHN)
Atlas des Zonages agricoles Hautes-Pyrénées Classement en zone défavorisée pour paiement de l'indemnité compensatoire Carte 1-3 de handicaps naturels (ICHN) Découpage infra-communaux Non défavorisée (65) SAINT CASTELNAU Zone défavorisée simple (112) LANNE RIVIERE-BASSE Piémont (57) Montagne (172) MADIRAN Haute montagne (85) HERES LABATUT SOUBLECAUSE RIVIERE HAGEDET CAUSSADE RIVIERE VILLEFRANQUE LASCAZERES ESTIRAC AURIEBAT SOMBRUN SAUVETERRE MAUBOURGUET LAHITTE TOUPIERE MONFAUCON LAFITOLE LARREULE VIDOUZE BUZON ANSOST NOUILHAN GENSAC BARBACHEN LIAC CAIXON SEGALAS ARTAGNAN SARRIAC RABASTENS VIC-EN-BIGORRE BIGORRE DE SANOUS BIGORRE VILLENAVE MINGOT ESTAMPURES PRES FRECHEDE BEARN SAINT-LEZER CAMALES BAZILLAC SAINT X SENAC SEVER MAZEROLLES U MOUMOULOUS ESCAUNETS A LACASSAGNE DE E BERNADETS D RUSTAN DEBAT VILLENAVE N UGNOUAS PUJO PRES O MARSAC C MANSAN FONTRAILLES S LESCURRY BOUILH TALAZAC E DEVANT MARSAC PEYRUN LAMEAC ANTIN SERON TOSTAT ANDREST GUIZERIX CASTERA TROULEY TRIE TARASTEIX SIARROUY SARNIGUET JACQUE LAPEYRE PEYRET LOU LABARTHE SUR BOUILH LUBRET AUBAREDE LARROQUE SAINT SAINT BAISE ANDRE CASTERETS AURENSAN SOREAC PEREUILH SADOURNIN LAGARDE GAYAN OSMETS LUC LALANNE TRIE SARIAC DOURS MARSEILLAN OROIX CHIS LOUIT MAGNOAC CHELLE LUBY VIDOU PUNTOUS BAZET DEBAT THERMES PINTAC COLLONGUES BETMONT PUYDARRIEUX OURSBELILLE CASTELNAU MAGNOAC GARDERES SABALOS CASTELVIEILH TOURNOUS MAGNOAC BOURS MUN DARRE N HACHAN BARTHE ORLEIX A N BETBEZE OLEAC LAMARQUE VILLEMBITS Z A DEBAT RUSTAING U N P ORGAN E BORDERES CABANAC P M S LALANNE LUQUET LUSTAR A SUR-L'ECHEZ -
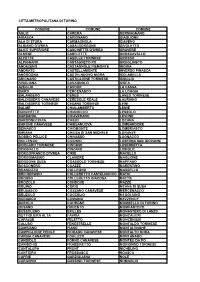
Città Metropolitana Di Torino Comune Comune Comune
CITTÀ METROPOLITANA DI TORINO COMUNE COMUNE COMUNE AGLIÈ CAREMA GERMAGNANO AIRASCA CARIGNANO GIAGLIONE ALA DI STURA CARMAGNOLA GIAVENO ALBIANO D'IVREA CASALBORGONE GIVOLETTO ALICE SUPERIORE CASCINETTE D'IVREA GRAVERE ALMESE CASELETTE GROSCAVALLO ALPETTE CASELLE TORINESE GROSSO ALPIGNANO CASTAGNETO PO GRUGLIASCO ANDEZENO CASTAGNOLE PIEMONTE INGRIA ANDRATE CASTELLAMONTE INVERSO PINASCA ANGROGNA CASTELNUOVO NIGRA ISOLABELLA ARIGNANO CASTIGLIONE TORINESE ISSIGLIO AVIGLIANA CAVAGNOLO IVREA AZEGLIO CAVOUR LA CASSA BAIRO CERCENASCO LA LOGGIA BALANGERO CERES LANZO TORINESE BALDISSERO CANAVESE CERESOLE REALE LAURIANO BALDISSERO TORINESE CESANA TORINESE LEINÌ BALME CHIALAMBERTO LEMIE BANCHETTE CHIANOCCO LESSOLO BARBANIA CHIAVERANO LEVONE BARDONECCHIA CHIERI LOCANA BARONE CANAVESE CHIESANUOVA LOMBARDORE BEINASCO CHIOMONTE LOMBRIASCO BIBIANA CHIUSA DI SAN MICHELE LORANZÈ BOBBIO PELLICE CHIVASSO LUGNACCO BOLLENGO CICONIO LUSERNA SAN GIOVANNI BORGARO TORINESE CINTANO LUSERNETTA BORGIALLO CINZANO LUSIGLIÈ BORGOFRANCO D'IVREA CIRIÈ MACELLO BORGOMASINO CLAVIERE MAGLIONE BORGONE SUSA COASSOLO TORINESE MAPPANO BOSCONERO COAZZE MARENTINO BRANDIZZO COLLEGNO MASSELLO BRICHERASIO COLLERETTO CASTELNUOVO MATHI BROSSO COLLERETTO GIACOSA MATTIE BROZOLO CONDOVE MAZZÈ BRUINO CORIO MEANA DI SUSA BRUSASCO COSSANO CANAVESE MERCENASCO BRUZOLO CUCEGLIO MEUGLIANO BURIASCO CUMIANA MEZZENILE BUROLO CUORGNÈ MOMBELLO DI TORINO BUSANO DRUENTO MOMPANTERO BUSSOLENO EXILLES MONASTERO DI LANZO BUTTIGLIERA ALTA FAVRIA MONCALIERI CAFASSE FELETTO MONCENISIO CALUSO FENESTRELLE MONTALDO -

Recensement ERP Annuel Par Commune Pour Site Prefecture
Recensement des Etablissements Recevant du Public Département du Gers AIGNAN Numéro Nom N° Adresse Type Catégorie Etat 136 ARENES ANDRE LADOUES AU VILLAGE PA 1 Ouvert 423 SALLE POLYVALENTE RUE DU BATAILLON DE L'ARMAGNAC L 3 Ouvert ESPACE D'ANIMATION 7710 INTERGENERATIONNEL PLACE DES ARENES L 3 Ouvert 3410 Salle des fêtes RUE SAINT SATURNIN L 4 Ouvert 213 COLLEGE VERT Route de Castelnavet R 4 Ouvert 213-100 COLLEGE VERT - INTERNAT CD N° 155 Rh 4 Ouvert 1052 Salle de réunions au stade Route de Blanin L 5 Ouvert 3411 Club House-Vestiaire du tennis VC n° 10 L 5 Ouvert 5570 Centre de Loisirs&Halte garderie Impasse des écoles L 5 Ouvert 6177 ESPA Place du Colonel Parisot L 5 Ouvert 7404 Club Associatif Moto -1, avenue des Pyrénées L 5 Ouvert 07862-000 Carrefour Proxi France 9 place du Colonel Parisot M 5 Ouvert 07918-000 Vente de fleurs et divers - KARINE FLEURS Place du Colonel PARISOT M 5 Ouvert 07922-000 Vignerons du Saint Mont - Cave d'Aignan Avenue de l'Armagnac M 5 Ouvert 07926-000 Coiffure plein Hair Rue du Duc de Bouillon M 5 Ouvert 07929-000 Coiffure Indiana Place du Colonel PARISOT M 5 Ouvert 3428 Boulangerie - M. et Mme JILET Rue Guillaume vital M 5 Ouvert 3535 Salon de thé-BROCANTHE "La belle Histoire" 14 place du Colonel Parisot M 5 Ouvert 6090 Pharmacie BEATRICE 9 rue du Duc de Bouillon M 5 Ouvert SARL BRICODARTI_Magasin de 7631 bricolage,jardinage Avenue du Docteur Dousset M 5 Ouvert 07924-000 Ferme auberge La Cave Lieu dit "La Cave" N 5 Ouvert 09887 LA FERME AUX BUFFLES LIEU-DIT LECTOURE N 5 Projet 3431 Café des sports "l'AIOLI"