Transport of Phenolic Compounds from Leaf Surface of Creosotebush and Tarbush to Soil Surface by Precipitation1
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Bull0664.Pdf (14.64Mb)
[Blank Page in Original Bulletin] Serious losses in sheep and goats as a result of grazing the ripe fruit of F_laurensia-cernua hm-kern observed on three ranches during the months of January and February. The characteristic pathological rlterations were inflammation, ulceration and perforation of the gastro- ' intestinal tract due to the presence ogsome intense irritant. In all cazes the animals had been subjected to cansiderable handling and were quite hungry when they gained access to the plant. When sheep and goats have continuous access to the plant and are not subjected to-handling during the winter months,there is no evidence that this p&k'of the ~lantis grazed in sufficient amounts to cause toxic effects. The plant has not been associated with similar losses in cattle. 1 The toxicity of the ripe fruit was demonstrated by experimental i feeding to sheep and goats. o In this wark a marked variation in the ~ueceptihility of different iddividuals was observed, as well as a nar- row margin between a sli$htly toxic andqethal dose of the material. I Lmses -frcm-this4ce can be avoided by preventing hungry animals from gainidg access to the plant during the winter months. There is no evidence that the green leaves constitute a hazard to livestock. 1 CONTENTS ~ Page 1 Introduction ...................................................... 5 Botanical Description and Distribution .............................. 7 Experimental Procedure ........................................... 8 C Feeding Ripe Fruit for One Day ............................... -

Sonoran Joint Venture Bird Conservation Plan Version 1.0
Sonoran Joint Venture Bird Conservation Plan Version 1.0 Sonoran Joint Venture 738 N. 5th Avenue, Suite 102 Tucson, AZ 85705 520-882-0047 (phone) 520-882-0037 (fax) www.sonoranjv.org May 2006 Sonoran Joint Venture Bird Conservation Plan Version 1.0 ____________________________________________________________________________________________ Acknowledgments We would like to thank all of the members of the Sonoran Joint Venture Technical Committee for their steadfast work at meetings and for reviews of this document. The following Technical Committee meetings were devoted in part or total to working on the Bird Conservation Plan: Tucson, June 11-12, 2004; Guaymas, October 19-20, 2004; Tucson, January 26-27, 2005; El Palmito, June 2-3, 2005, and Tucson, October 27-29, 2005. Another major contribution to the planning process was the completion of the first round of the northwest Mexico Species Assessment Process on May 10-14, 2004. Without the data contributed and generated by those participants we would not have been able to successfully assess and prioritize all bird species in the SJV area. Writing the Conservation Plan was truly a group effort of many people representing a variety of agencies, NGOs, and universities. Primary contributors are recognized at the beginning of each regional chapter in which they participated. The following agencies and organizations were involved in the plan: Arizona Game and Fish Department, Audubon Arizona, Centro de Investigación Cientifica y de Educación Superior de Ensenada (CICESE), Centro de Investigación de Alimentación y Desarrollo (CIAD), Comisión Nacional de Áreas Naturales Protegidas (CONANP), Instituto del Medio Ambiente y el Desarrollo (IMADES), PRBO Conservation Science, Pronatura Noroeste, Proyecto Corredor Colibrí, Secretaría de Medio Ambiente y Recursos Naturales (SEMARNAT), Sonoran Institute, The Hummingbird Monitoring Network, Tucson Audubon Society, U.S. -

Flourensia Cernua DC: a Plant from Mexican Semiarid Regions with a Broad Spectrum of Action for Disease Control
27 Flourensia cernua DC: A Plant from Mexican Semiarid Regions with a Broad Spectrum of Action for Disease Control Diana Jasso de Rodríguez1, F. Daniel Hernández-Castillo1, Susana Solís-Gaona1, Raúl Rodríguez- García1 and Rosa M. Rodríguez-Jasso2 1Universidad Autónoma Agraria Antonio Narro (UAAAN), Buenavista, Saltillo, Coahuila 2Centre of Biological Engineering, University of Minho,Campus Gualtar 1México 2Portugal 1. Introduction Mexico has an extensive variety of plants, it is the world´s fourth richest country in this aspect. Some 25,000 species are registered, and it is thought that there are approximately 30, 000 not described. Particularly the regions of the north of Mexico, with their semiarid climate, have a great number and variety of wild plants grown under extreme climatic conditions. Wild species which have compounds with flavonoid structures, sesquiterpenoids, acetylenes, p-acetophenones, benzofurans, and benzopyrans grow in these regions. The polyphenolic compounds include tannins and flavonoids which have therapeutic uses due to their anti-inflammatory, antifungal, antibacterial, antioxidant, and healing properties. Flourensia cernua is an endemic species which grows in semiarid zones of Mexico and contains polyphenolic, lactone, benzofuran, and benzopyran compounds which give it a potential use for disease control. In this work, F. cernua is reviewed in terms of its geographical distribution in Mexico, traditional uses, bioactive compounds identified for controlling fungi, bacteria, and insects, as well as cytotoxic activity. 2. Common names It is commonly known in different ways, as it is found in the United States of America as well as in Mexico. The names given in the United States of America are: tarbush, hojase, American-tarbush, black-brush, varnish-brush, and hojasen (Correl and Johnston, 1970; Vines, 1960). -

A Preliminary Vegetation Classification of the Tombstone, Arizona, Vicinity
AN ABSTRACT OF THE THESIS OF EDMUNDO GARCIA-MOYA for theDOCTOR OF PHILOSOPHY (Name) (Degree) ANIMAL SCIENCE in (RANGELAND RESOURCES) presented onfi,?,(2;2-01) / Yj) Qi (Major) (Date) Title: A PRELIMINARY VEGETATION CLASSIFICATION OF THE TOMBSTONE, ARIZONA, VICINITY Redacted for Privacy Abstract approved: C. E. Poulton The need for classifying vegetation in a more precise wayis evident.Also, there is a need to provide a hierarchicalclassification scheme that will match changes in image characteristics as one moves through the scales from spaceto conventional aerial photo- graphy.Such refined classifications of vegetation are thefirst steps toward a better understanding of the potentialities andlimitations of a specified area which help in the detection of analogousenvironmental conditions for resource allocation and management purposes.These needs become more urgent as use and competitionfor natural resources and land increases.The first approximation of a classification scheme may meet these needs for a test site inthe Tombstone, Arizona, vicinity.This classification task was accomplished by the use of a "hierarchical- polythetic-agglomerative" package using presence-absence data and standardized cover esti- mates, The following tentative associations and a variant were found upon division of the original data into groups of convenient size to meet the limitations of the programs: Association A (Panicum hirticaule/Tidestromia lanuginosa- Boerhaavia coulteri) la (a variant of Association A) Association B (Rhus microphylla-Dalea formosa) -

New Mexico Range Plants
New Mexico Range Plants Circular 374 Revised by Christopher D. Allison and Nick Ashcroft1 Cooperative Extension Service • College of Agricultural, Consumer and Environmental Sciences New Mexico contains almost 78 million acres, more than 90 percent of which is in native vegetation grazed by domestic livestock and wildlife. The kinds of plants that grow on a range, along with their quality and quan- tity, determine its value. A successful rancher knows the plants on his or her range. There are more than 3,000 species of plants in New Mexico. The 85 discussed here are most important to the livestock industry. Most of these are native plants. RANGELAND AREAS OF NEW MEXICO Figure 1 represents the major rangeland areas in New Mexico. The northern desert, western plateau, and high valley areas are enough alike to be described together, as are the central and high plains areas and the southern desert and basin. Southern Desert and Basin 36 - New Mexico and Arizona Plateaus and Mesas 37 - San Juan River Valley, mesas and Plateaus The southern desert and basin occupies much of south- 39 - Arizona and New Mexico Mountains 41 - Southeastern Arizona Basin and Range 42 - Southern Desertic Basins, Plains and Mountains ern New Mexico at elevations between 3,000 and 5,000 48 - Southern Rocky Mountains 51 - High Intermountain Valleys feet. This area follows the Rio Grande north into the 70 - Pecos/Canadian Plains and Valleys southern part of Sandoval County. 77 - Southern High Plains Some of the most common plants are creosote bush (Larrea tridentata [DC.] Coville), mesquite (Prosopis Figure 1. -
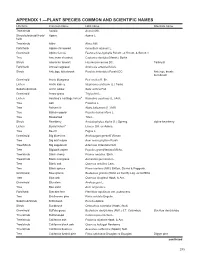
APPENDIX 1.—PLANT SPECIES COMMON and SCIENTIFIC NAMES Life Form Common Name Latin Name Alternate Name Tree/Shrub Acacia Acacia Mill
APPENDIX 1.—PLANT SPECIES COMMON AND SCIENTIFIC NAMES Life form Common name Latin name Alternate name Tree/shrub Acacia Acacia Mill. Shrub/subshrub//Forb/ Agave Agave L. herb Tree/shrub Alder Alnus Mill. Forb/herb Alpine chickweed Cerastium alpinum L. Graminoid Alpine fescue Festuca brachyphylla Schult. ex Schult. & Schult. f. Tree American chestnut Castanea dentata (Marsh.) Borkh. Shrub American tarwort Flourensia cernua DC. Tarbrush Forb/herb Annual ragweed Ambrosia artemisiifolia L. Shrub Antelope bitterbrush Purshia tridentata (Pursh) DC. Antelope brush; buckbrush Graminoid Arctic bluegrass Poa arctica R. Br. Lichen Arctic kidney Nephroma arcticum (L.) Torss. Subshrub/shrub Arctic willow Salix arctica Pall. Graminoid Arrow grass Triglochin L. Lichen Asahina’s cartilage lichend Ramalina asahinae (L.) Ach. Tree Ash Fraxinus L. Tree Balsam fi r Abies balsamea (L.) Mill. Tree Balsam poplar Populus balsamifera L. Tree Basswood Tilia L. Shrub Bearberry Arctostaphylos alpina (L.) Spreng. alpine bearberry Lichen Beard lichena Usnea Dill. ex Adans. Tree Beech Fagus L. Graminoid Big bluestem Andropogon gerardii Vitman Tree Big leaf maple Acer macrophyllum Pursh Tree/Shrub Big sagebrush Artemisia tridentata Nutt. Tree Bigtooth aspen Populus grandidentata Michx. Tree/shrub Black cherry Prunus serotina, Ehrh. Tree/shrub Black mangrove Avicennia germinans L. Tree Black oak Quercus velutina, Lam. Tree Black spruce Picea mariana (Mill.) Britton, Sterns & Poggenb. Graminoid Blue grama Bouteloua gracilis (Willd. ex Kunth) Lag. ex Griffi ths Tree Blue oak Quercus douglasii Hook. & Arn. Graminoid Bluestem Andropogon L. Tree Box elder Acer negundo L. Forb/herb Bracken fern Pteridium aquilinum var. pubescens Tree Bristlecone pine Pinus aristata Engelm. Subshrub/Shrub Brittlebush Encelia Adans. -

Proceedings: Shrubland Dynamics -- Fire and Water
Ecology and Population Biology Session Estimating Aboveground Biomass of Mariola (Parthenium incanum) from Plant Dimensions Carlos Villalobos1 Abstract: The distribution and abundance of plant biomass in space and time are important properties of rangeland ecosystem. Land managers and researchers require reliable shrub weight estimates to evaluate site productivity, food abundance, treatment effects, and stocking rates. Rapid, nondestructive methods are needed to estimate shrub biomass in semi-arid ecosystems. Shrub height and crown diameter are useful non-destructive measures of shrub size. Mariola (Parthenium incanum) is an important shrub that is widely distributed in the Chihuahuan and Sonoran deserts. Mariola is found from the southwest United States to the central part of Mexico. Regression analyses were used to examine the relationships between aboveground biomass and four plant measurements (shrub height, longest canopy width, shortest canopy width, and crown volume) from 45 plants. All variables were related to aerial biomass; R values varied from 0.73 to 0.98. Regression equations developed for mariola compared favorably to equations in similar species in desert environments, suggesting that results might be applicable to other desert regions for rapid and accurate estimation of shrub biomass. Introduction goldeneye (Viguiera cordifolia), and ocotillo (Fouquieria splendens). Among these shrubs, fourwing saltbush The Chihuahuan desert is the largest of the three creosote- (Atriplex canescens) and mariola (Parthenium incanum) bush-dominated deserts in North America. The Chihuahuan are important components of the diet of grazing animals. desert covers 450,000 to 629,000 km2 (Henrickson and Maynez and others (1984) evaluated the nutritional value Straw 1976; Morafka 1977; Dinerstein and others 2000) of mariola by collecting samples during 1 year in a monthly in eastern Chihuahua, western Coahuila, San Luis, Potosi, period. -

Proceedings: Shrubland Ecosystem Dynamics in a Changing Environment; 1995 May 23-25; Las Cruces, NM
This file was created by scanning the printed publication. Errors identified by the software have been corrected; however, some errors may remain. Tarbush Leaf Surface Terpene Profile in Relation to Mammalian Herbivory R. E. Estell E. L. Fredrickson D. M. Anderson K. M. Havstad M. D. Remmenga Abstract-Cattle, sheep and goats were densely stocked in pad herbivory by various mammals (Schwartz and others 1980; docks containing tarbush (Flourensia cernua DC) for six to nine Reichardt and others 1985; Elliott and Loudon 1987; days and defoliation of 160 plants was recorded daily during two Bucyanayandi and others 1990; Goralka and Langenheim years. Plants were separated into high, moderate or low defoliation 1991; Zhang and States 1991). categories. Leaves were collected from plants during the same stage Differential defoliation oftarbush (Flourensia cernua DC) of maturity during the third year. Leaf surface compounds were when browsed simultaneously by cattle, sheep and goats in extracted with ethanol and mono- and sesquiterpenes were ana a previous study at this location was related to concentration lyzed using gas chromatography/ion trap mass spectrometry. A set of epicuticular wax and two unidentified terpenes (Estell of 11 variables was identified that appeared most closely related to and others 1994a). Tarbush contains several classes of plant defoliation categories: dry matter, ash, limonene, camphor, secondary compounds (Kingston and others 1975; Dillon borneol, a copaene, B caryophyllene, a pinene, p-cymene, cis-jasmone and others 1976; Bohlmann and Grenz 1977; Aregullin and caryophyllene oxide concentrations. This group distinguished Gallardo 1985). While relatively unpalatable, tarbush leaves among all three defoliation categories (P < 0.03) when subjected to can be consumed safely in moderate amounts for several multivariate analysis. -

Jackrabbit (Lepus Californicus) Herbivory Changes Dominance in Desertified Chihuahuan Desert Ecosystems
ARTICLE IN PRESS Journal of Arid Environments Journal of Arid Environments 70 (2007) 418–426 www.elsevier.com/locate/jaridenv Jackrabbit (Lepus californicus) herbivory changes dominance in desertified Chihuahuan Desert ecosystems G.A. Rotha,b, W.G. Whitfordb,Ã, Y. Steinbergera aFaculty of Life Sciences, Bar-Ilan University, Israel bUSDA-ARS Jornada Experimental Range (JER), MSC 3JER New Mexico State University, Las Cruces, N.M. 88003, USA Received 21 August 2006; received in revised form 2 January 2007; accepted 29 January 2007 Available online 23 March 2007 Abstract This study addressed the question: can herbivory by a medium size herbivore, black-tail jackrabbits (Lepus californicus), change dominance in desertified ecosystems dominated by two species of shrubs. Shrubs that were pruned by jackrabbits in plant communities dominated by creosotebush (Larrea tridentata) and tarbush (Flourensia cernua) were compared to shrubs not browsed by the rabbits. In the mixed shrub area, herbivory on F. cernua resulted in death of 46.6% of the shrubs, compared to only 4.8% of L. tridentata shrubs. There was no evidence of jackrabbit browsing of dead F. cernua in a tarbush monoculture area. The canopy volumes of F. cernua plants that survived repeated browsing were significantly smaller than predicted based on unbrowsed plants with the same basal stem areas. Jackrabbit browsing resulted in increased canopy volume of creosotebush shrubs. Creosotebush average canopy volume significantly exceeded predicted values because of compensatory growth of stems from nodes below the severed point. Close spatial association of lightly browsed creosotebushes with heavily browsed tarbush may be a factor contributing to low utilization of creosotebush stems by jackrabbits. -

Age and Body Condition of Goats Influence Consumption of Juniper and Monoterpene-Treated Feed
Age and Body Condition of Goats Influence Consumption of Juniper and Monoterpene-Treated Feed Item Type text; Article Authors Frost, Rachel A.; Launchbaugh, Karen L.; Taylor, Charles A. Citation Frost, R. A., Launchbaugh, K. L., & Taylor, C. A. (2008). Age and body condition of goats influence consumption of juniper and monoterpene-treated feed. Rangeland Ecology & Management, 61(1), 48-54. DOI 10.2111/06-160R1.1 Publisher Society for Range Management Journal Rangeland Ecology & Management Rights Copyright © Society for Range Management. Download date 23/09/2021 16:50:36 Item License http://rightsstatements.org/vocab/InC/1.0/ Version Final published version Link to Item http://hdl.handle.net/10150/642924 Rangeland Ecol Manage 61:48–54 | January 2008 Age and Body Condition of Goats Influence Consumption of Juniper and Monoterpene-Treated Feed Rachel A. Frost,1 Karen L. Launchbaugh,2 and Charles A. Taylor, Jr.3 Authors are 1Post Doctoral Research and Extension Associate, Animal and Range Sciences Department, Montana State University, Bozeman, MT 59717; 2Associate Professor, Department of Rangeland Ecology and Management, University of Idaho, Moscow, ID 83844; and 3Professor, Texas Agricultural Experiment Station, Sonora, TX 76950. Abstract Redberry juniper (Juniperus pinchotii Sudworth) is an invasive, evergreen tree that is rapidly expanding throughout western and central Texas. Goats will consume some juniper on rangelands; however, intake is limited. The objective of our research was to determine how the age and body condition of goats influence their consumption of juniper and an artificial feed containing 4 monoterpenes. Two separate experiments were conducted. Experiment 1 examined the intake of redberry juniper foliage and used 39 goats either young (2 yr) or mature (. -

Using Landform and Vegetative Factors to Improve the Interpretation of Landsat Lm-Agery
MELVINSAITERWHITE U.S. Army Engineer Topographic Laboratories Fort Belvoir, VA 22060 WILLIAMF~CE JEROMESHIPMAN IB M Federal Systems Division Gaithersburg, MD 20879 Using Landform and Vegetative Factors to Improve the Interpretation of Landsat lm-agery Land-cover units associated with major landform conditions were readily classified with reasonable accuracy to level 3 and at times to level 4. INTRODUCTION have been classified by this method for agricultural LASSIFYING land cover and land use by applying and forest inventories and geological prospecting. C pattern classification algorithms to digital mul- In particular, investigators have evaluated this use tispectral sensor data has become increasingly of multispectral scanner digital data in order to map common since the technique was first proposed (Fu and inventory forested and rangeland resources. ABSTRACT:A general classification of land uselland cover to level 2 can be obtained from Landsat data by using supervised digital classification techniques. However, because dqferent land-cover classes often fall into the same spectral class, a finer level of detail cannot be readily achieved in the Landsat image ry. The purpose of this study was to evaluate digitally classified Landsat imagery and to determine those vegetative and terrain factors that could aid in the interpretation of the imagery. A methodology was developed for comparing descriptive landform and land-cover ground truth data with the corresponding digital imagery which ex- ploited knowledge of (I) plant community and landform relations, (2) the species phenological and physiognomic characteristics, and (3) the reflectance-cover re- lationships. Using this methodology, land-cover units associated with major landform con- ditions were readily classified with reasonable accuracy to level 3 and at times to level 4. -

Defoliation of Flourensia Cernua (Tarbush) with High-Density Mixed- Species Stocking
Journal of Arid Environments 130 (2016) 62e67 Contents lists available at ScienceDirect Journal of Arid Environments journal homepage: www.elsevier.com/locate/jaridenv Defoliation of Flourensia cernua (tarbush) with high-density mixed- species stocking * R.E. Estell , D.M. Anderson, D.K. James USDA-ARS, Jornada Experimental Range, Las Cruces, NM 88003-0003, USA article info abstract Article history: Interest in shrub use by livestock is increasing along with the rising demands placed on rangelands Received 2 September 2015 worldwide. Historically, Flourensia cernua (tarbush) has increased in the Chihuahuan Desert but receives Received in revised form limited use by cattle. Cattle, sheep and goats co-grazed eight 0.6 ha tarbush-dominated paddocks during 25 March 2016 two periods for up to nine days during two consecutive years. Cumulative tarbush defoliation across Accepted 26 March 2016 periods and years averaged 75.6%, with a mean increase of 9.3%/day (P < 0.0001). Defoliation of indi- Available online 31 March 2016 vidual shrubs varied from 5 to 99% in 1989 and 0e100% in 1990, indicating highly variable palatability among individual plants. Sheep lost 2.3e5.5 kg/hd (P < 0.0001) across periods and years when forced to Keywords: ¼ Biocontrol browse tarbush. In 1989, goats gained (P 0.0345) 0.6 kg/hd in period 1, but the gain in period 2 was not fi ¼ ¼ Browsing signi cant (P 0.2934). During 1990, goats lost 3.1 kg/hd (P 0.0001) across periods. High-density Cattle mixed-species stocking of small areas for short time periods resulted in extensive tarbush use, primar- Goats ily due to browsing by sheep and goats.