Discovery and Distribution of Root Lesion Nematode
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Nematodes in Potato Soils in New Brunswick J Kimpinski I and EM
Canadian Plant Disease Survey 68:2,1988 147 Nematodes in potato soils in New Brunswick J Kimpinski I and EM. Smith2 Root-lesion nematodes (Pratylenchus spp.) were the dominant plant-parasitic nematodes in potato fields in the Grand Falls region of New Brunswick, Canada. Pratylenchus crenatus was more prevalent than P. penetrans. The northern root-knot nematode (Meloidogyne hapla) and clover-cyst nematode (Hetemdera trifolid were not detected in the survey. Can. Plant Dis. Surv. 68:2. 147-148, 1988. Dans des champs de pommes de terre de la region de Grand Falls au Nouveau-Brunswick (Canada), les principaux nematodes parasites des vegetaux identifies Btaient des nematodes radicicoles (Pratylenchus spp.). On a signale plus de Pratylenchus crenatus que de P. penetrans. On n'a pas trouve de nematode cecidogbne du nord (Meloidogyne hapla) ou de nematode B kyste du trefle (Heterodera trifolid au cours de I'enquste. Introduction and counted, and other nematode genera were identified with a stereomicroscope at 60 X Extracted nematodes were pre- A nematode survey conducted in 1979 in the Grand Falls . served in 5% formalin and up to 100 nematodes from each region of New Brunswick indicated that root-lesion nematodes sample were selected randomly and examined at 1000 X with (Pratylenchus crenatus Loof and P. penetrans (Cobb) Filipjev a compound microscope. and Sch. Stek.) were the dominant species of plant-parasitic nematodes in potato roots and soils (4).It was also determined that population levels of the northern root-knot nematode Results (Meloidogyne hapla Chitwood) were very low, being detected Root-lesion nematodes were the dominant plant-parasitic in only 5% of the root and soil samples. -

Investigation of the Development of Root Lesion Nematodes, Pratylenchus Spp
Türk. entomol. derg., 2021, 45 (1): 23-31 ISSN 1010-6960 DOI: http://dx.doi.org/10.16970/entoted.753614 E-ISSN 2536-491X Original article (Orijinal araştırma) Investigation of the development of root lesion nematodes, Pratylenchus spp. (Tylenchida: Pratylenchidae) in three chickpea cultivars Kök lezyon nematodlarının, Pratylenchus spp. (Tylenchida: Pratylenchidae) üç nohut çeşidinde gelişmesinin incelenmesi İrem AYAZ1 Ece B. KASAPOĞLU ULUDAMAR1* Tohid BEHMAND1 İbrahim Halil ELEKCİOĞLU1 Abstract In this study, penetration, population changes and reproduction rates of root lesion nematodes, Pratylenchus neglectus (Rensch, 1924), Pratylenchus penetrans (Cobb, 1917) and Pratylenchus thornei Sher & Allen, 1953 (Tylenchida: Pratylenchidae), at 3, 7, 14, 21, 28, 35, 42, 49 and 56 d after inoculation in chickpea Bari 2, Bari 3 (Cicer reticulatum Ladiz) and Cermi [Cicer echinospermum P.H.Davis (Fabales: Fabaceae)] were assessed in a controlled environment room in 2018-2019. No juveniles were observed in the roots in the first 3 d after inoculation. Although, population density of P. thornei reached the highest in Cermi (21 d), Bari 3 (42 d) and the lowest observed on Bari 2. Pratylenchus neglectus reached the highest population density in Bari 3 and Cermi on day 28. The population density of P. neglectus was the lowest in Bari 2. Also, population density of P. penetrans reached the highest in Bari 3 cultivar within 49 d, similar to P. thornei, whereas Bari 2 and Cermi had low population densities during the entire experimental period. Keywords: -

Description of Pratylenchus Dunensis Sp. N. (Nematoda: Pratylenchidae
Nematology, 2006, Vol. 8(1), 79-88 Description of Pratylenchus dunensis sp.n.(Nematoda: Pratylenchidae), a root-lesion nematode associated with the dune grass Ammophila arenaria (L.) Link ∗ Eduardo DE LA PEÑA 1, , Maurice MOENS 1,2, Adriaan VA N AELST 3 and Gerrit KARSSEN 4,5 1 Agricultural Research Centre, Crop Protection Department, Burg. van Gansberghelaan 96, 9820, Merelbeke, Belgium 2 Gent University, Laboratory for Agrozoology, Coupure 653, 9000 Gent, Belgium 3 Wageningen University & Research Centre, Laboratory of Plant Cell Biology, Arboretumlaan 4, 6703 BD Wageningen, The Netherlands 4 Plant Protection Service, Nematology Section, P.O. Box 9102, 6700 HC Wageningen, The Netherlands 5 Wageningen University & Research Centre, Laboratory of Nematology, Binnenhaven 5, 6709 PD Wageningen, The Netherlands Received: 4 April 2005; revised: 7 November 2005 Accepted for publication: 7 November 2005 Summary – A root-lesion nematode, Pratylenchus dunensis sp. n., is described and illustrated from Ammophila arenaria (L.) Link, a grass occurring abundantly in coastal dunes of Atlantic Europe. The new species is characterised by medium sized (454-579 µm) slender, vermiform, females and males having two lip annuli (sometimes three to four; incomplete incisures only visible with scanning electron microscopy), medium to robust stylet (ca 16 µm) with robust stylet knobs slightly set off, long pharyngeal glands (ca 42 µm), lateral field with four parallel, non-equidistant, lines, the middle ridge being narrower than the outer ones, lateral field with partial areolation and lines converging posterior to the phasmid which is located between the two inner lines of the lateral field in the posterior half of the tail, round spermatheca filled with round sperm, vulva at 78% of total body length and with protruding vulval lips, posterior uterine sac relatively short (ca 19 µm), cylindrical tail (ca 33 µm) narrowing in the posterior third with smooth tail tip and with conspicuous hyaline part (ca 2 µm). -

"Structure, Function and Evolution of the Nematode Genome"
Structure, Function and Advanced article Evolution of The Article Contents . Introduction Nematode Genome . Main Text Online posting date: 15th February 2013 Christian Ro¨delsperger, Max Planck Institute for Developmental Biology, Tuebingen, Germany Adrian Streit, Max Planck Institute for Developmental Biology, Tuebingen, Germany Ralf J Sommer, Max Planck Institute for Developmental Biology, Tuebingen, Germany In the past few years, an increasing number of draft gen- numerous variations. In some instances, multiple alter- ome sequences of multiple free-living and parasitic native forms for particular developmental stages exist, nematodes have been published. Although nematode most notably dauer juveniles, an alternative third juvenile genomes vary in size within an order of magnitude, com- stage capable of surviving long periods of starvation and other adverse conditions. Some or all stages can be para- pared with mammalian genomes, they are all very small. sitic (Anderson, 2000; Community; Eckert et al., 2005; Nevertheless, nematodes possess only marginally fewer Riddle et al., 1997). The minimal generation times and the genes than mammals do. Nematode genomes are very life expectancies vary greatly among nematodes and range compact and therefore form a highly attractive system for from a few days to several years. comparative studies of genome structure and evolution. Among the nematodes, numerous parasites of plants and Strikingly, approximately one-third of the genes in every animals, including man are of great medical and economic sequenced nematode genome has no recognisable importance (Lee, 2002). From phylogenetic analyses, it can homologues outside their genus. One observes high rates be concluded that parasitic life styles evolved at least seven of gene losses and gains, among them numerous examples times independently within the nematodes (four times with of gene acquisition by horizontal gene transfer. -

The Effect of Pratylenchus Zeae on the Growth and Yield of Upland Rice
The effect of Pratylenchus zeae on the growth and yield of upland rice Richard A. PLOWRIGHT",Danilo MATUS**, Tin AUNG**and Twng-Wah MEW** * CAB International Institute of Parasitology, 395 a, Hatfield Road, St. Albans, Hertfordshire, AL4 OXU, UK and ** International Rice Research Institute, P. O. Box 933, Manila, Philippines. SUMMARY The root lesion nematode Pratylenchus zeae is widely distributed on upland rice but its economic importance has not been assessed. In a field trial, following a five month clean fallow, the ofcontrol P. zeae using carbofuran, increased the yieldof cv. Upl Ri-5 whilst the yield of cv. Kinandang Patong was unaffected. Pre-sowing soil population densities (Pi) of P. zeae were low (0-1 11 nematodes/lOOml soil) and there were no obvious symptoms of infectionduring early vegetativegrowth although the plant height of Upl Ri-5 was slightly reduced. At harvest the yield of treated plants was increased byO/O 13-29of that of untreated plants having a mean infection of 1 350 nematodedg root(P < 0.05). In the glasshouse the rate of growth and tilleringof cv. IR36 was significantly reduced with a highPi (630-3 O00 nematodesllO0 cm3 soil). Infected root systems were stunted and mean root fresh weight was reduced by 40-60%. Although infection reducedthe no. of spikeletslplant, these plants had a higher harvest index and consequently grain yield was unaffected. The relationship between yield and the population density of P. zeae at different crop growth stages, in the field indicates low tolerance and a high relative minimum yield of 65O/o. RESUMÉ Influence de Pratylenchus zeae sur la croissance et la récolte du riz de plateau Pratylenchus zeae est très répandusur le riz de plateau mais son importance n'a jamais été évaluée. -

Diversity, Phylogeny, Characterization and Diagnostics of Root-Knot and Lesion Nematodes
Diversity, phylogeny, characterization and diagnostics of root-knot and lesion nematodes Toon Janssen Promotors: Prof. Dr. Wim Bert Prof. Dr. Gerrit Karssen Thesis submitted to obtain the degree of doctor in Sciences, Biology Proefschrift voorgelegd tot het bekomen van de graad van doctor in de Wetenschappen, Biologie 1 Table of contents Acknowledgements Chapter 1: general introduction 1 Organisms under study: plant-parasitic nematodes .................................................... 11 1.1 Pratylenchus: root-lesion nematodes ..................................................................................... 13 1.2 Meloidogyne: root-knot nematodes ....................................................................................... 15 2 Economic importance ..................................................................................................... 17 3 Identification of plant-parasitic nematodes .................................................................. 19 4 Variability in reproduction strategies and genome evolution ..................................... 22 5 Aims .................................................................................................................................. 24 6 Outline of this study ........................................................................................................ 25 Chapter 2: Mitochondrial coding genome analysis of tropical root-knot nematodes (Meloidogyne) supports haplotype based diagnostics and reveals evidence of recent reticulate evolution. 1 Abstract -

Description of Pratylenchus Gutierrezi N. Sp. (Nematoda: Pratylenchidae
Journal of Nematology 24(2):298-304. 1992. © The Society of Nematologists 1992. Description of Pratylenchus 9utierrezi n. sp. (Nematoda: Pratylenchidae) from Coffee in Costa Rica A. MORGAN GOLDEN, 1 ROGER L6PEZ CH., 2 AND HERNAN VILCHEZ R. 2 Abstract: A lesion nematode, Pratylenchu6 gutierrezi n. sp., collected from the roots of coffee in the Central Plateau of Costa Rica, is described and illustrated. Its relationships to Pratylenchusflakkensis, P. similis, and P. gibbicaudatus, the only other species of the genus having two head annules, males, or spermatheca with sperm, and an annulated tail terminus, is discussed. Other distinctive characters are its posterior vulva (mean of 80%); its prominently rounded stylet knobs, low head, and subcy- lindrical tail. SEM observations provide additional details of females and males, especially face views, which show for the first time sexual dimorphism. Key words: Coffea arabica, Costa Rica, lesion nematode, morphology, nematode, new species, Pra- tylenchus flakkensis, P. gibbicaudatus, P. gutierrezi n. sp., P. similis, scanning electron microscopy, SEM, taxonomy. Certain species of lesion nematodes to be a new Pratylenchus species, which is (Pratylenchus spp.) are important parasites described and illustrated herein. of coffee (Coffea spp.). In a recent excellent review of nematodes reported to occur on MATERIALS AND METHODS coffee (1), the following five Pratylenchus species were listed: P. brachyurus (Godfrey, Nematodes were extracted from in- 1929) Filipjev & Schuurmans Stekhoven, fected coffee roots by placing chopped or 1941; P. coffeae (Zimmermann, 1889) Fil- blenderized roots on filter paper over wa- ipjev & Schuurmans Stekhoven, 1941; P. ter in a Baermann funnel. Specimens were goodeyi Sher & Alien, 1953; P. -

Root-Lesion Nematodes: Biology and Management in Pacific Northwest Wheat Cropping Systems Richard W
A Pacific Northwest Extension Publication Oregon State University • University of Idaho • Washington State University PNW 617 • October 2015 Root-lesion nematodes: Biology and management in Pacific Northwest wheat cropping systems Richard W. Smiley ematodes are microscopic but complex symptoms on small grain cereals are nonspecific unsegmented roundworms that are anatomi- and easily confused with other ailments such as Ncally differentiated for feeding, digestion, nitrogen deficiency, low water availability, and root locomotion, and reproduction. These small animals rots caused by fungi such as Pythium, Rhizoctonia, occur worldwide in all environments. Most species and Fusarium. Farmers, pest management advi- are beneficial to agriculture; they make important sors, and scientists routinely underestimate or fail to contributions to organic matter decomposition recognize the impact of root-lesion nematodes on and are important members of the soil food chain. wheat. It is now estimated that these root parasites However, some species are parasitic to plants or reduce wheat yields by about 5 percent annually in animals. each of the Pacific Northwest (PNW) states of Idaho, Plant-parasitic nematodes in the genus Oregon, and Washington. This generally unrecog- Pratylenchus are commonly called either root-lesion nized pest annually reduces wheat profitability by as nematodes or lesion nematodes. These parasites much as $51 million in the PNW. can be seen only with the aid of a microscope. They Description are transparent, eel-shaped, and about 1/64 inch (0.5 mm) long. They puncture root cells and There are nearly 70 species in the genus damage underground plant tissues. Feeding by these Pratylenchus, at least eight of which are parasitic to nematodes reduces plant vigor, causes lesions, and wheat. -

The Root Lesion Nematodes of Banana: Pratylenchus Coffeae, Pratylenchus Goodeyi
Back to Musa pest fact sheets Musa Pest Fact Sheet No. 2 THE ROOT LESION NEMATODES OF BANANA Pratylenchus coffeae (Zimmermann, 1898) Filip. & Schu. Stek., 1941 Pratylenchus goodeyi Sher & Allen, 1953 John Bridge, Roger Fogain and Paul Speijer (November 1997) • • • • • •• ••• •• • •• • ••• • •• • • •• ••• • •• • •• • • • • • • • • • • • • • Pratylenchus coffeae • Pratylenchus goodeyi Worldwide distribution of Pratylenchus goodeyi and P. coffeae (after J. Bridge) The root lesion nematodes Pr a tylenchus coffeae a n d C e n t ral and South America it is the most important Pr a tylenchus goodeyi are both major pests of M u s a nematode species affecting Cavendish cultivars (Musa wherever they occur. The damage they cause is very AAA) in Honduras. In Africa, although it is widespread similar to that caused by the other important banana and important in South Africa and in Ghana, where it is root nematode pest Radopholus similis. reported to cause up to 60% production loss in the plant crop of plantains (M u s a AAB), in other A f r i c a n Distribution countries its distribution is very localized indicating that it is probably a recent introduction here. In West P. coffeae is probably a native of the Pacific and Pacific Africa, P. coffeae generally occurs in mixed populations Rim countries but now has a world wide distribution, with Helicotylenchus multicinctus, Radopholus similis almost equal to that of R. similis although more clus- and Meloidogyne spp. This nematode species has an tered (see map). It is most likely that it has been spread extremely wide host range and is also a major pest on around the world on commercial banana planting other crops such as yams, ginger, turmeric, abaca and material. -

Functional Diversity of Soil Nematodes in Relation to the Impact of Agriculture—A Review
diversity Review Functional Diversity of Soil Nematodes in Relation to the Impact of Agriculture—A Review Stela Lazarova 1,* , Danny Coyne 2 , Mayra G. Rodríguez 3 , Belkis Peteira 3 and Aurelio Ciancio 4,* 1 Institute of Biodiversity and Ecosystem Research, Bulgarian Academy of Sciences, 2 Y. Gagarin Str., 1113 Sofia, Bulgaria 2 International Institute of Tropical Agriculture (IITA), Kasarani, Nairobi 30772-00100, Kenya; [email protected] 3 National Center for Plant and Animal Health (CENSA), P.O. Box 10, Mayabeque Province, San José de las Lajas 32700, Cuba; [email protected] (M.G.R.); [email protected] (B.P.) 4 Consiglio Nazionale delle Ricerche, Istituto per la Protezione Sostenibile delle Piante, 70126 Bari, Italy * Correspondence: [email protected] (S.L.); [email protected] (A.C.); Tel.: +359-8865-32-609 (S.L.); +39-080-5929-221 (A.C.) Abstract: The analysis of the functional diversity of soil nematodes requires detailed knowledge on theoretical aspects of the biodiversity–ecosystem functioning relationship in natural and managed terrestrial ecosystems. Basic approaches applied are reviewed, focusing on the impact and value of soil nematode diversity in crop production and on the most consistent external drivers affecting their stability. The role of nematode trophic guilds in two intensively cultivated crops are examined in more detail, as representative of agriculture from tropical/subtropical (banana) and temperate (apple) climates. The multiple facets of nematode network analysis, for management of multitrophic interactions and restoration purposes, represent complex tasks that require the integration of different interdisciplinary expertise. Understanding the evolutionary basis of nematode diversity at the field Citation: Lazarova, S.; Coyne, D.; level, and its response to current changes, will help to explain the observed community shifts. -

Pratylenchus
Pratylenchus Taxonomy Class Secernentea Order Tylenchida Superfamily Tylenchoidea Family Pratylenchidae Genus Pratylenchus The genus name is derived from the words pratum (Latin= meadow), tylos (Greek= knob) and enchos ( Greek=spear). Originally described as Tylenchus pratensis by De Man in 1880 from a meadow in England. Pratylenchus scribneri was reported from potato in Tennessee in 1889. Root-lesion nematodes of the genus Pratylenchus are recognised worldwide as major constraints of important economic crops, including banana, cereals, coffee, corn, legumes, peanut, potato and many fruits. Their economic importance in agriculture is due to their wide host range and their distribution in every terrestrial environment on the planet (Castillo and Vovlas, 2007). Plant‐parasitic nematodes of the genus Pratylenchus are among the top three most significant nematode pests of crop and horticultural plants worldwide. There are more than 70 described species, most are polyphagous with a wide range of host plants. Because they do not form obvious feeding patterns characteristic of sedentary endoparasites (e.g. galls or cysts), and all worm‐like stages are mobile and can enter and leave host roots, it is more difficult to recognise their presence and the damage they cause. Morphology There are more than 70 described species, fewer than half of them are known to have males. Morphological identification of Pratylenchus species is difficult, requiring considerable subjective evaluation of characters and overlapping morphomertrics. Nematodes in this genus are 0.4-0.5 mm long (under 0.8 mm). No sexual dimorphism in the anterior part of the body. Deirids absent. Lip area low, flattened anteriorly, not offset, or only weakly offset, from body contour. -
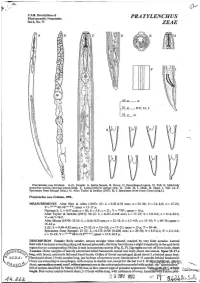
Pratylenchus Zeae
/p C.I.H. Descriptions of Plant-parasitic Nematodes PRATYLENCHUS Set 6, No. 77 ZEAE ' A D F V L 50 P A i5 ,B-F,H,I 25P ,G G Prutylenchus zeue Graham. A-G. Female. A. Entire female. B. Ovary. C. Oesophageal region. D. Tail. E. Mid-body ' transverse section showing lateral fields. F. Lateral field in surface view. G. Tails. H, I. Male. H. Head. I. Tail. (A-F. Specimens from Senegal (orig.). G. After Taylor & Jenkins (1957). H, I. Specimens from Ivory Coast (orig.).) PratyZemhus zeae Graham, 1951. MEASUREMENTS After Sher & Allen (1953): 99: L = 0.36-0.58 mm; a = 25-30; b = 5.4-8.0; c= 17-21; V = 26-43 68-763.4-6."; spear = 15-17 p. Neotype 9: L = 0.47 mm; a = 26; b = 5.9; c = 21 ; V = 30704;spear = 16 p. After Taylor & Jenkins (1957): 90 99: L = 0.413-0.639 mm; a = 17-25; b = 5.0-9.6; c = 11.2-24.1; v = 64.7-74.9. After Memy (1970): 25 99: L = 0.34-0.55 mm; a = 22-33; b = 3.3-4.9; c = 13-18; V = 69-74; spear = 15-18 p. 5 88: L = 0.40-0.42 mm; a = 27-32; b = 3.6-5.0; c = 17-21 ;spear = 15 p; T = 30-44. Specimens from Senegal: 25 99: L = 0.373-0.506 (0.428) mm; a = 20-30; b = 4.9-6.1 ; b' = 3.2-4.6; c = 15-19; V = 23-38 68.6-73.93-86.7; spear= 15.5-16.5 p.