Zika Viral Infection
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Arizona Arboviral Handbook for Chikungunya, Dengue, and Zika Viruses
ARIZONA ARBOVIRAL HANDBOOK FOR CHIKUNGUNYA, DENGUE, AND ZIKA VIRUSES 7/31/2017 Arizona Department of Health Services | P a g e 1 Arizona Arboviral Handbook for Chikungunya, Dengue, and Zika Viruses Arizona Arboviral Handbook for Chikungunya, Dengue, and Zika Viruses OBJECTIVES .............................................................................................................. 4 I: CHIKUNGUNYA ..................................................................................................... 5 Chikungunya Ecology and Transmission ....................................... 6 Chikungunya Clinical Disease and Case Management ............... 7 Chikungunya Laboratory Testing .................................................. 8 Chikungunya Case Definitions ...................................................... 9 Chikungunya Case Classification Algorithm ............................... 11 II: DENGUE .............................................................................................................. 12 Dengue Ecology and Transmission .............................................. 14 Dengue Clinical Disease and Case Management ...................... 14 Dengue Laboratory Testing ......................................................... 17 Dengue Case Definitions ............................................................ 19 Dengue Case Classification Algorithm ....................................... 23 III: ZIKA .................................................................................................................. -

Zika Virus Outside Africa Edward B
Zika Virus Outside Africa Edward B. Hayes Zika virus (ZIKV) is a flavivirus related to yellow fever, est (4). Serologic studies indicated that humans could also dengue, West Nile, and Japanese encephalitis viruses. In be infected (5). Transmission of ZIKV by artificially fed 2007 ZIKV caused an outbreak of relatively mild disease Ae. aegypti mosquitoes to mice and a monkey in a labora- characterized by rash, arthralgia, and conjunctivitis on Yap tory was reported in 1956 (6). Island in the southwestern Pacific Ocean. This was the first ZIKV was isolated from humans in Nigeria during time that ZIKV was detected outside of Africa and Asia. The studies conducted in 1968 and during 1971–1975; in 1 history, transmission dynamics, virology, and clinical mani- festations of ZIKV disease are discussed, along with the study, 40% of the persons tested had neutralizing antibody possibility for diagnostic confusion between ZIKV illness to ZIKV (7–9). Human isolates were obtained from febrile and dengue. The emergence of ZIKV outside of its previ- children 10 months, 2 years (2 cases), and 3 years of age, ously known geographic range should prompt awareness of all without other clinical details described, and from a 10 the potential for ZIKV to spread to other Pacific islands and year-old boy with fever, headache, and body pains (7,8). the Americas. From 1951 through 1981, serologic evidence of human ZIKV infection was reported from other African coun- tries such as Uganda, Tanzania, Egypt, Central African n April 2007, an outbreak of illness characterized by rash, Republic, Sierra Leone (10), and Gabon, and in parts of arthralgia, and conjunctivitis was reported on Yap Island I Asia including India, Malaysia, the Philippines, Thailand, in the Federated States of Micronesia. -

Overview of Zika
Overview of Zika Alan D.T. Barrett Department of Pathology Sealy Center for Vaccine Development University of Texas Medical Branch Galveston TX Disclaimer • Over 700 papers in pubmed on Zika in the last 12 months. • Impossible to stay up to date as field is moving so fast. • Only including material in public domain. ZIKV: 1947-2006 • Zika virus (ZIKV) causes Zika fever (ZF), an acute febrile illness characterized by a rash, conjunctival injection, arthralgia, myalgia and headache. • The disease appears in all age groups with an incubation period on the order of 3-14 days and a symptomatic phase lasting about 2-7 days. • Treatment is largely symptomatic. • The illness is mild in nature with a very low rate of hospitalization. • The vast majority of patients make a full recovery and while death is rarely reported, it has primarily occurred in the immunocompromised or those with other complicating medical conditions. • Only 14 clinical cases in the literature from 1951-2006. Map showing the known distribution of Zika virus based on serosurveys, virus detection, and laboratory-diagnosed cases. Blue arrows show recent patterns of spread deduced from phylogenetic studies Weaver et al Antiviral Research, Volume 130, 2016, 69–80 ZIKV in the Americas Current ZIKV activity ZIKV transmission • ZIKV is primarily transmitted by Aedes spp. Mosquitoes. • Infectious ZIKV particles have been demonstrated in urine, saliva, semen, blood products, and breast milk possible vehicles of transmission. • Male-to-female and male-to-male transmission of ZIKV have been reported, but only from individuals with clinical signs/symptoms of ZIKV infection. • Establishing person-to-person transmission is difficult, as contacts frequently have the same environmental exposures. -

Zika Virus E an Overview
+ MODEL Microbes and Infection xx (2016) 1e7 www.elsevier.com/locate/micinf Review Zika virus e an overview Camila Zanluca, Claudia Nunes Duarte dos Santos* Laboratorio de Virologia Molecular, Instituto Carlos Chagas, Fundaçao~ Oswaldo Cruz, Curitiba, PR, Brazil Received 14 February 2016; accepted 3 March 2016 Available online ▪▪▪ Abstract Zika virus (ZIKV) is currently one of the most important emerging viruses in the world. Recently, it has caused outbreaks and epidemics, and has been associated with severe clinical manifestations and congenital malformations. However to date, little is known about the pathogenicity of the virus and the consequences of ZIKV infection. In this paper, we provide an overview of the current knowledge on ZIKV. © 2016 Institut Pasteur. Published by Elsevier Masson SAS. All rights reserved. Keywords: Zika virus; Flavivirus; Arthropod-borne virus; Viral emergence 1. Introduction 2. Epidemiology Zika virus (ZIKV) is an arthropod-born virus (arbovirus) Zika virus was first isolated in 1947 from the serum of a belonging to the genus Flavivirus and the family Flaviviridae sentinel Rhesus monkey from the Zika Forest (Uganda) during [1]. The virus carries the name of the forest where it was first a study on the transmission of yellow fever. The second identified [2], a name that means “overgrown” in the Luganda isolation was made from a pool of Aedes (Stegomyia) africa- language [3]. nus mosquitoes from the same forest in 1948 [2]. The occur- Beyond ZIKV, the genus Flavivirus comprises 52 other rence of human infection was first evidenced by the presence viral species, including the dengue, yellow fever, Saint Louis of neutralising antibodies in the sera of east African residents encephalitis and West Nile viruses [1]. -

Dengue Fever/Severe Dengue Fever/Chikungunya Fever! Report on Suspicion of Infection During Business Hours
Dengue Fever/Severe Dengue Fever/Chikungunya Fever! Report on suspicion of infection during business hours PROTOCOL CHECKLIST Enter available information into Merlin upon receipt of initial report Review background information on the disease (see Section 2), case definitions (see Section 3 for dengue and for chikungunya), and laboratory testing (see Section 4) Forward specimens to the Florida Department of Health (DOH) Bureau of Public Health Laboratories (BPHL) for confirmatory laboratory testing (as needed) Inform local mosquito control personnel of suspected chikungunya or dengue case as soon as possible (if applicable) Inform state Arbovirus Surveillance Coordinator on suspicion of locally acquired arbovirus infection Contact provider (see Section 5A) Interview case-patient Review disease facts (see Section 2) Mode of transmission Ask about exposure to relevant risk factors (see Section 5. Case Investigation) History of travel, outdoor activities, and mosquito bites two weeks prior to onset History of febrile illness or travel for household members or other close contacts in the month prior to onset History of previous arbovirus infection or vaccination (yellow fever, Japanese encephalitis) Provide education on transmission and prevention (see Section 6) Awareness of mosquito-borne diseases Drain standing water at least weekly to stop mosquitoes from multiplying Discard items that collect water and are not being used Cover skin with clothing or Environmental Protection Agency (EPA)-registered repellent such as DEET (N,N-diethyl-meta-toluamide) Use permethrin on clothing (not skin) according to manufacturer’s directions Cover doors and windows with intact screens to keep mosquitoes out of the house Enter additional data obtained from interview into Merlin (see Section 5D) Arrange for a convalescent specimen to be taken (if necessary) Dengue/Chikungunya Guide to Surveillance and Investigation Dengue Fever/Severe Dengue/Chikungunya 1. -
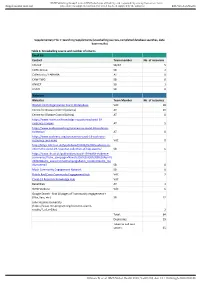
Supplementary File 1: Searching Supplements (Snowballing Sources, Completed Database Searches, Data Base Results)
BMJ Publishing Group Limited (BMJ) disclaims all liability and responsibility arising from any reliance Supplemental material placed on this supplemental material which has been supplied by the author(s) BMJ Global Health Supplementary File 1: Searching Supplements (snowballing sources, completed database searches, data base results) Table 1: Snowballing source and number of returns Email list Contact Team member No. of resources CH-CoP SB/AT 5 CORE Group SB 2 Collectivity / FARAFRA AT 0 CHW-TWG SB 0 UNICEF SB 1 USAID SB 0 Websites Websites Team Member No. of resources World Health Organization Covid-19 database VdC 18 Centre for Disease Control (Atlanta) AT 10 Centre for Disease Control (Africa) AT 0 https://www.nccmt.ca/knowledge-repositories/covid-19- evidence-reviews AT 5 https://www.evidenceaid.org/coronavirus-covid-19-evidence- collection/ AT 0 https://www.cochrane.org/coronavirus-covid-19-cochrane- resources-and-news VdC 0 http://blogs.lshtm.ac.uk/hppdebated/2020/04/08/evidence-to- inform-the-covid-19-response-collection-of-hpp-papers/ SB 6 https://www.ids.ac.uk/publications/covid-19-health-evidence- summaries/?utm_campaign=News%20at%20IDS%208%20April% 202020&utm_source=emailCampaign&utm_content=&utm_me dium=email SB 0 Mesh Community Engagement Network SB 0 British Red Cross Community Engagement Hub VdC 2 Covid-19 Research Knowledge Hub VdC ReliefWeb AT 3 WHO Website VdC 6 Google Search - first 10 pages of "community engagement + (Zika, Sars, etc) SB 12 John Hopkins University (https://www.mcsprogram.org/resource-search- results/?_sf_s=Zika) 2 Total: 64 Duplicates: 29 Taken to Full text screen: 35 Gilmore B, et al. -

EDITORIAL Zika Virus
EDITORIAL Bangladesh Medical Journal Khulna Zika virus - A global concern Maternal infection with Zika virus during the first trimester of pregnancy increases the risk of microcephaly in the baby. In late 2015, there were reports of a dramatic increase in the prevalence Vol. 49 No. 1 & 2 June & December 2016 of microcephaly in Brazil, coinciding with an outbreak of the Zika virus several months earlier. The WHO declared a Public The Journal is published twice a year in the Health Emergency of International Concern on 28 January 2016, months of June and December keeping in view the lesson learnt from the African Ebola outbreak.1 On March 22, 2016, WHO Director-General briefs the media that the status of Zika has changed from a mild medical curiosity to a disease with severe public health implications in less than a year. Subsequently, a causal association was acknowledged by WHO and Centers for Disease Control and Prevention (CDC) in April, 2016. With the steady increase in microcephaly, the Brazilian Ministry of Health set up a surveillance system and upto June 4, 2016, 7830 suspected cases 2 had been reported. Editorial Board Epidemiology: Zika virus (ZiV) continues to spread geographically to areas where competent vectors are present. Editor Although a decline in cases of Zika infection has been reported in Choudhury Habibur Rasul some countries, in later parts of the year, vigilance needs to remain high. Seventy-five countries and territories have reported Members evidence of mosquito-borne Zika virus transmission which are 3 SK Ballav divided in three categories. Md Mahmud Hassan The Zika virus outbreak made an unprecedented health crisis with Kazi Hafizur Rahman enormous health and social costs. -

Zika Incident Response Playbook
Zika Incident Response Playbook 2017 Zika Incident Response Playbook Zika Incident Response Playbook Table of Contents HAZARD OVERVIEW ……………………………………………………… PAGE 2 Transmission Symptoms Florida Risk Assessment INCIDENT MANAGEMENT STRUCTURE …………………………………… PAGE 14 INCIDENT OBJECTIVES ………………………………………………….. PAGE 15 Overarching Incident Priorities Phase 1: Imported Cases with Vector Present, No Local Transmission Phase 2: Confirmed Local Transmission in Florida, Single Case / Cluster Phase 3: Widespread Local Transmission within Single County Phase 4: Widespread Local Transmission in Multiple Counties ESSENTIAL ELEMENTS OF INFORMATION ……………………………….. PAGE 25 ADVANCED PLANNING CONSIDERATIONS ..……………………………... PAGE 26 SUPPORTING PLANS AND PROCEDURES ……………………………… PAGE 29 PRE-SCRIPTED MESSAGES……………………………………………… PAGE 31 Version 4.0 February, 2017 Page 1 Zika Incident Response Playbook HAZARD OVERVIEW Zika fever is a febrile illness caused by a mosquito-borne virus similar to those that cause dengue and West Nile virus infection. Since May 2015, local Zika virus transmission has been identified in throughout the Americas, including Puerto Rico, Texas and Florida. Outbreaks have previously been reported in Africa, Southeast Asia, and the Pacific Islands. Cases of Zika fever continue to be reported in travelers returning to the continental United States. Zika has been associated with fetal abnormalities, specifically microcephaly. Microcephaly is a birth defect where a baby’s head is smaller than expected when compared to babies of the same sex and age. Babies with microcephaly often have smaller brains that might not have developed properly. Adverse pregnancy and infant outcomes associated with Zika virus infection during pregnancy are being studied. Zika has also been associated with neurological complications, including Guillain-Barré syndrome (GBS). GBS is a rare disorder in which a person’s immune system damages their nerve cells, causing muscle weakness and sometimes paralysis. -

High Dose Intravenous Vitamin C Treatment for Zika Fever
JOM Volume 31, Number 1, 2016 Case Report 19 High Dose Intravenous Vitamin C Treatment for Zika Fever Michael J. Gonzalez, NMD, DSc, PhD, FACN;1 Miguel J. Berdie,l MD;2 Jorgé R. Miranda- Massari, PharmD;2 Jorgé Duconge, PhD;1 Joshua L. Rodríguez-López, BS;3 Pedro A. Adrover-López, BS 3 ¹University of Puerto Rico, Medical Sciences Campus, Schools of Public Health and Pharmacy, San Juan, PR, 00936-5067 ²Berdiel Clinic, Ponce, PR, 00716 3Ponce Health Sciences University, School of Medicine, Ponce, PR, 00716 Abstract !e Zika Fever is a viral disease caused by a single-stranded RNA virus from the Fla- vivirus genus, Flaviviridae family, from the Spondweni group. Its transmission occurs through mosquito vectors, principally Aedes Aegypti. !e most common symptoms of Zika are fever, rash, joint pain, and conjunctivitis (red eyes). Other common symptoms include muscle pain and headache. As of now, no vaccine exists for the virus and no o"cial treatment has been developed aside from standard procedures of the use of acetaminophen (paracetamol) and non-steroidal anti-in#ammatory drugs. !is is a case report of a 54 year-old Hispanic female who arrived at the clinic with symptomatology congruent with the Zika fever. !e patient was treated with high doses of intravenous vitamin C over three days. !e symptoms resolved after the infusions without any side e$ects at day four. Re- covery from this viral infection takes normally around two weeks. Based on the positive outcome in this case, we propose that intravenous vitamin C should be studied further as a potential treatment for acute viral infections. -

August 2019 Vol 25, No 8, August 2019
® August 2019 Pregnancy and Maternal Health Pregnancy Vol 25, No 8, August 2019 EMERGING INFECTIOUS DISEASES Pages 1445–1624 DEPARTMENT OF HEALTH & HUMAN SERVICES MEDIA MAIL Public Health Service POSTAGE & FEES PAID Centers for Disease Control and Prevention (CDC) Mailstop D61, Atlanta, GA 30329-4027 PHS/CDC Official Business Permit No. G 284 Penalty for Private Use $300 Return Service Requested Gift of George N. and Helen M. Richard, 1964. Image © The Metropolitan Museum of Art. Image source: Art Resource, NY Resource, Art source: Image Art. of Museum Metropolitan The © Image 1964. Richard, M. Helen and N. George of Gift . Oil on canvas; 28 1/2 in x 35 7/8 in/72.4 cm x 91.1 cm. cm. 91.1 x cm in/72.4 7/8 35 x in 1/2 28 canvas; on Oil . (1890) First Steps, after Millet after Steps, First Vincent van Gogh (1853–1890). (1853–1890). Gogh van Vincent ISSN 1080-6040 Peer-Reviewed Journal Tracking and Analyzing Disease Trends Pages 1445–1624 EDITOR-IN-CHIEF D. Peter Drotman ASSOCIATE EDITORS EDITORIAL BOARD Paul M. Arguin, Atlanta, Georgia, USA Barry J. Beaty, Fort Collins, Colorado, USA Charles Ben Beard, Fort Collins, Colorado, USA Martin J. Blaser, New York, New York, USA Ermias Belay, Atlanta, Georgia, USA Christopher Braden, Atlanta, Georgia, USA David M. Bell, Atlanta, Georgia, USA Arturo Casadevall, New York, New York, USA Sharon Bloom, Atlanta, Georgia, USA Kenneth G. Castro, Atlanta, Georgia, USA Richard Bradbury, Atlanta, Georgia, USA Vincent Deubel, Shanghai, China Mary Brandt, Atlanta, Georgia, USA Christian Drosten, Charité Berlin, Germany Corrie Brown, Athens, Georgia, USA Isaac Chun-Hai Fung, Statesboro, Georgia, USA Charles H. -

EMERGING INFECTIOUS DISEASES : Actions Needed to Address
United States Government Accountability Office Report to Congressional Requesters May 2017 EMERGING INFECTIOUS DISEASES Actions Needed to Address the Challenges of Responding to Zika Virus Disease Outbreaks GAO-17-445 May 2017 EMERGING INFECTIOUS DISEASES Actions Needed to Address the Challenges of Responding to Zika Virus Disease Outbreaks Highlights of GAO-17-445, a report to congressional requesters. Why GAO Did This Study What GAO Found Zika virus disease can cause adverse Since Zika virus disease was a newly emerging disease threat in the United pregnancy and neurological outcomes. States, and relatively little was known about the Zika virus prior to the 2016 U.S. Given this ongoing threat, GAO was outbreak, the Centers for Disease Control and Prevention (CDC), and the states asked to evaluate progress made and were not fully equipped with needed information and resources at the beginning challenges faced by federal agencies of the outbreak. This presented several challenges for Zika virus disease in responding to the Zika virus surveillance and research efforts, such as challenges related to establishing a outbreak in the United States. national definition for reporting cases. Knowledge about Zika virus epidemiology GAO examined (1) information on what has increased in the past year, including information about Zika virus disease is known and not known about the incidence and distribution of cases, and its associated adverse health outcomes. epidemiology of the Zika virus, and any Most of the 5,197 Zika virus disease cases reported by April 5, 2017 in the challenges with conducting United States were associated with travel from affected areas outside the surveillance and epidemiological continental United States. -

Structure of a Key Protein from the Zika Virus
BIOLOGICAL SCIENCES Structure of a Key Protein from the Zika Virus Zika’s Emergence In 1947, scientists working on a yellow-fever research program in Uganda, Africa, recorded a tempera- ture spike in one of their “sentinel” rhesus monkeys (no. 766). It had been kept in a strip of densely forested area along the edge of Lake Victoria called the Zika Forest. The monkey soon recovered, showing no other symptoms. The scientists isolated the agent that caused the fever and named it the “Zika virus.” Over the next 60 years, fewer than 20 human Comparison of the solved ZIKV NS5 structure to those of the Japanese encephalitis virus (JEV) and Zika infections were recorded, the dengue fever virus (DENV). perhaps because infection was associated with only mild effects. The Zika virus (ZIKV) is a mosqui- three proteins that are part of the virus The first large outbreak of Zika fever to-borne pathogen recently linked to itself (structural proteins) and seven occurred in 2007 in Micronesia, with certain birth defects in infants in South proteins that are not part of the virus, but 49 confirmed cases, underscoring and Central America and the United could perform some viral function in the Zika’s potential as a newly emerging States. Protein crystallography studies infected cell (nonstructural proteins). mosquito-borne virus. In 2013, a larger epidemic occurred in French performed at the ALS have now resolved In ZIKV, nonstructural protein 5 (NS5) Polynesia, with an estimated 30,000 the structure of a key ZIKV protein (NS5) includes two domains: one that facilitates symptomatic infections, including to 3.0 Å.