Gonadotropins Regulate Rat Testicular Tight Junctions in Vivo
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Kisspeptin and Testicular Function—Is It Necessary?
International Journal of Molecular Sciences Review Kisspeptin and Testicular Function—Is It Necessary? Aditi Sharma 1 , Thilipan Thaventhiran 1, Suks Minhas 2, Waljit S. Dhillo 1 and Channa N. Jayasena 1,* 1 Section of Investigative Medicine, Imperial College, 6th Floor, Commonwealth Building, Hammersmith Hospital, 150 Du Cane Road, London W12 0NN, UK; [email protected] (A.S.); [email protected] (T.T.); [email protected] (W.S.D.) 2 Department of Urology, Imperial College Healthcare NHS Trust, Charing Cross Hospital, Fulham Palace Road, Hammersmith, London W6 8RF, UK; [email protected] * Correspondence: [email protected] Received: 12 March 2020; Accepted: 21 April 2020; Published: 22 April 2020 Abstract: The role of kisspeptin in stimulating hypothalamic GnRH is undisputed. However, the role of kisspeptin signaling in testicular function is less clear. The testes are essential for male reproduction through their functions of spermatogenesis and steroidogenesis. Our review focused on the current literature investigating the distribution, regulation and effects of kisspeptin and its receptor (KISS1/KISS1R) within the testes of species studied to date. There is substantial evidence of localised KISS1/KISS1R expression and peptide distribution in the testes. However, variability is observed in the testicular cell types expressing KISS1/KISS1R. Evidence is presented for modulation of steroidogenesis and sperm function by kisspeptin signaling. However, the physiological importance of such effects, and whether these are paracrine or endocrine manifestations, remain unclear. Keywords: kisspeptin; kisspeptin receptor; spermatozoa; Leydig cells; Sertoli cells; testes; testosterone; LH; FSH; spermatogenesis 1. Introduction Kisspeptin is an established regulator of puberty onset [1,2], sexual maturation and adult reproductive activity [3]. -

Mouse Model of Male Germ Cell Apoptosis in Response to a Lack of Hormonal Stimulation
Indian Journal of Experimental Biology Vol. 43, November 2005, pp. 1048-1057 Mouse model of male germ cell apoptosis in response to a lack of hormonal stimulation Ami ya P Sinha Hikim*, Yanira Vera, Rashid I Elhag, Yanhe Lue, Yu-Gui Cui , Vanisha Pope, Andrew Leun g, Vince Atienza, Christina Wan g & Ron ald S Swerdloff Di vision of Endocrinology, Department of Medicine, Harbor-UCLA Medical Center, David Geffen School of Medicine at UCLA and Los Angeles Biomedical Research Institute, Torrance. Californi a. USA Received 5 August 2005 As a prerequisite for studies using mutant mi ce, we established a mouse model for induction of male germ ce ll apoptosis after depri vation of gonadotropins and intratesti c ul ar testosterone (T). We employed a potent long acting gonadotropin-releasing hormone antagoni st (GnRH-A), acyline, al one or in combinati on with an anti and rogen, flutamide for effective inducti on of germ cell apoptosis in mice. Combined treatment with continuous release of acyline (3 mg/kg BW/day) with flutamide (in the form of sc pellets of 25 mg) resul ted in almost th e same level of suppression of spermatogenesis, as judged by testi s weight and by germ cell apoptotic index, in 2 weeks as th at re ported for rats after treatment with 1.25 mg/kg BW Nai-Giu GnRH-A for the same time peri od. Within the study paradi gm, the maximum suppression of spermatogenesis occurred after a single sc injecti on of hi gh (20 mg/kg BW) dose of acyli ne with flutamide. -

The Effect of Gonadotropin Withdrawal and Stimulation with Human Chorionic Gonadotropin on Intratesticular Androstenedione and DHEA in Normal Men
ORIGINAL ARTICLE Endocrine Research The Effect of Gonadotropin Withdrawal and Stimulation with Human Chorionic Gonadotropin on Intratesticular Androstenedione and DHEA in Normal Men M. Y. Roth, S. T. Page, K. Lin, B. D. Anawalt, A. M. Matsumoto, B. Marck, W. J. Bremner, and J. K. Amory Downloaded from https://academic.oup.com/jcem/article/96/4/1175/2720870 by guest on 02 October 2021 Departments of Internal Medicine (M.Y.R., S.T.P., B.D.A., A.M.M., W.J.B., J.K.A.) and Obstetrics and Gynecology (K.L.) and Center for Research in Reproduction and Contraception (M.Y.R., S.T.P., B.D.A., A.M.M., W.J.B., J.K.A.), University of Washington, Seattle, Washington 91895; and Geriatric Research (B.M.), Education and Clinical Center, Veterans Affairs Puget Sound Health Care System, Seattle, Washington 98105 Introduction: Concentrations of intratesticular (IT) testosterone (T) are known to be 100–200 times those of serum T; however, the IT concentrations of T’s precursors, their testicular to serum gra- dients, gonadotropin dependence, and response to stimulation with human chorionic gonado- tropin (hCG) have not been studied in detail. We hypothesized that serum and IT androstenedione (ADD) and IT dehydroepiandrosterone (DHEA) would be significantly suppressed by the adminis- tration of a GnRH antagonist and increased when stimulated by hCG, without a similar suppression of serum DHEA. Methods: We suppressed gonadotropins in 23 normal men with the GnRH antagonist acyline and randomly assigned them to one of four doses of hCG, 0, 15, 60, or 125 IU sc every other day for 10 d. -

Suppression of Kisspeptin Expression and Gonadotropic Axis Sensitivity Following Exposure to Inhibitory Day Lengths in Female Siberian Hamsters
Hormones and Behavior 52 (2007) 492–498 www.elsevier.com/locate/yhbeh Suppression of kisspeptin expression and gonadotropic axis sensitivity following exposure to inhibitory day lengths in female Siberian hamsters Alex O. Mason a, Timothy J. Greives b, Melissa-Ann L. Scotti b, Jacob Levine a, Stefanie Frommeyer b, Ellen D. Ketterson b, ⁎ Gregory E. Demas b, Lance J. Kriegsfeld a, a Department of Psychology and Helen Wills Neuroscience Institute, 3210 Tolman Hall, MC 1650, University of California, Berkeley, Berkeley, CA 94720-1650, USA b Department of Biology, Center for the Integrative Study of Animal Behavior and Program in Neuroscience, Indiana University, Bloomington, IN 47405, USA Received 10 May 2007; revised 7 July 2007; accepted 10 July 2007 Available online 21 July 2007 Abstract To avoid breeding during unsuitable environmental or physiological circumstances, the reproductive axis adjusts its output in response to fluctuating internal and external conditions. The ability of the reproductive system to alter its activity appropriately in response to these cues has been well established. However, the means by which reproductively relevant cues are interpreted, integrated and relayed to the reproductive axis remain less well specified. The neuropeptide kisspeptin has been shown to be a potent positive stimulator of the hypothalamo–pituitary–gonadal (HPG) axis, suggesting a possible neural locus for the interpretation/integration of these cues. Because a failure to inhibit reproduction during winter would be maladaptive for short-lived female rodents, female Siberian hamsters (Phodopus sungorus) housed in long and short days were examined. In long “summer” photoperiods, kisspeptin is highly expressed in the anteroventral periventricular nucleus (AVPV), with low expression in the arcuate nucleus (Arc). -

Acyline: the First Study in Humans of a Potent, New Gonadotropin-Releasing Hormone Antagonist
0013-7227/02/$15.00/0 The Journal of Clinical Endocrinology & Metabolism 87(7):3215–3220 Printed in U.S.A. Copyright © 2002 by The Endocrine Society Acyline: The First Study in Humans of a Potent, New Gonadotropin-Releasing Hormone Antagonist KAREN L. HERBST, BRADLEY D. ANAWALT, JOHN K. AMORY, AND WILLIAM J. BREMNER Department of Medicine, University of Washington (K.L.H., B.D.A., J.K.A., W.J.B.) and Medical Service, Department of Veteran Affairs, Puget Sound Health Care System (B.D.A.), Seattle, Washington 98195 Acyline is a novel GnRH antagonist found in animal studies to maintaining suppression for over 48 h. Serum acyline levels -be a potent suppressor of circulating gonadotropin and tes- peaked at1hat18.9 ؎ 0.9 ng/ml, remained significantly ele tosterone (T) levels. We conducted the first study of acyline vated above background 7 d after injection, and returned to administration to humans. Eight healthy, eugonadal young background levels by 14–17 d after injection. Side-effects at men were administered a series of acyline injections (0, 2.5, the site of injection were limited to infrequent blush and pru- 7.5, 25, and 75 g/kg), each injection separated by at least 10 d. ritus that resolved within 90 min of injection. Higher doses of Serum FSH, LH, and T levels were measured for 7 d after acyline might be effective as depot injections for long-lasting injections. Acyline suppressed FSH, LH, and T levels in a dose- gonadotropin suppression in hormone-dependent diseases dependent fashion. Maximal suppression occurred after in- and for use in male hormonal contraception regimens. -

A Focus on the Kisspeptin Receptor, Kiss1r
Western University Scholarship@Western Electronic Thesis and Dissertation Repository 12-1-2014 12:00 AM Pathway-Specific Signaling and its Impact on erF tility: A Focus on the Kisspeptin Receptor, Kiss1r Maryse R. Ahow The University of Western Ontario Supervisor Dr. Andy Babwah The University of Western Ontario Graduate Program in Physiology A thesis submitted in partial fulfillment of the equirr ements for the degree in Doctor of Philosophy © Maryse R. Ahow 2014 Follow this and additional works at: https://ir.lib.uwo.ca/etd Part of the Molecular and Cellular Neuroscience Commons Recommended Citation Ahow, Maryse R., "Pathway-Specific Signaling and its Impact on erF tility: A Focus on the Kisspeptin Receptor, Kiss1r" (2014). Electronic Thesis and Dissertation Repository. 2537. https://ir.lib.uwo.ca/etd/2537 This Dissertation/Thesis is brought to you for free and open access by Scholarship@Western. It has been accepted for inclusion in Electronic Thesis and Dissertation Repository by an authorized administrator of Scholarship@Western. For more information, please contact [email protected]. PATHWAY-SPECIFIC SIGNALING AND ITS IMPACT ON FERTILITY: A FOCUS ON THE KISSPEPTIN RECEPTOR, Kiss1r (Thesis format: Monograph) by Maryse R. Ahow Graduate Program in Physiology A thesis submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy The School of Graduate and Postdoctoral Studies The University of Western Ontario London, Ontario, Canada © Maryse R. Ahow, 2014 Abstract Hypothalamic gonadotropin-releasing hormone (GnRH) is the master regulator of the neuroendocrine reproductive (HPG) axis and its secretion is regulated by various afferent inputs to the GnRH neuron. -

Therapeutic Implications for Castration-Resistant Prostate Cancer
Research Article Intraprostatic Androgens and Androgen-Regulated Gene Expression Persist after Testosterone Suppression: Therapeutic Implications for Castration-Resistant Prostate Cancer Elahe A. Mostaghel,1,2 Stephanie T. Page,2,5 Daniel W. Lin,3,5 Ladan Fazli,6 Ilsa M. Coleman,1 Lawrence D. True,4 Beatrice Knudsen,1 David L. Hess,7 Colleen C. Nelson,6 Alvin M. Matsumoto,2,5 William J. Bremner,2 Martin E. Gleave,6 and Peter S. Nelson1 1Fred Hutchinson Cancer Research Center; Departments of 2Medicine, 3Urology, and 4Pathology, University of Washington School of Medicine; 5Veterans Affairs Puget Sound Health Care System, Seattle, Washington; 6Vancouver General Hospital, Vancouver, British Columbia, Canada; and 7Oregon National Primate Research Center, Beaverton, Oregon Abstract efficacy will require testing of novel approaches targeting complete suppression of systemic and intracrine contribu- Androgen deprivation therapy (ADT) remains the primary tions to the prostatic androgen microenvironment. treatment for advanced prostate cancer. The efficacy of ADT [Cancer has not been rigorously evaluated by demonstrating suppres- Res 2007;67(10):5033–41] sion of prostatic androgen activity at the target tissue and molecular level. We determined the efficacy and consistency of Introduction medical castration in suppressing prostatic androgen levels Androgens are important mediators of transcriptional pathways and androgen-regulated gene expression. Androgen levels and controlling the proliferation, differentiation, and apoptosis of androgen-regulated -

(12) Patent Application Publication (10) Pub. No.: US 2010/0190692 A1 VAN GROENINGHEN (43) Pub
US 20100190692A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2010/0190692 A1 VAN GROENINGHEN (43) Pub. Date: Jul. 29, 2010 (54) METHODS FOR REDUCING Publication Classification GNRH-POSITIVE TUMIORCELL (51) Int. Cl. PROLIFERATION A638/09 (2006.01) (76) Inventor: JOHANNES C. VAN A638/22 (2006.01) GROENINGHEN, Dortmund (DE) A 6LX 3/59 (2006.01) s A63L/704 (2006.01) Correspondence Address: Age, 30.8; } GRUND INTELLECTUAL PROPERTY GROUP NKOLAISTRASSE 15 (52) U.S. Cl. .............. 514/8: 514/15; 514/259.5; 514/34; (57) ABSTRACT (21) Appl. No.: 12/701,284 A method for recognizing and evaluating the presence and (22) Filed: Feb. 5, 2010 function of GnRH receptors on tumor cells originating in the brain and/or nervous system and/or the meninges and/or reac Related U.S. Application Data tive neuroglia cells and/or primitive neuroectodermal tumor cells and/or on Kaposi sarcoma is provided. Furthermore a (63) Continuation-in-part of application No. 10/327,621, method for reducing degenerate GnRH-positive tumor cells filed on Dec. 20, 2002, now Pat. No. 7,695,722, which and/or for decreasing cellular replication of the above GnRH is a continuation-in-part of application No. 09/446, positive tumor cells comprising administering to a cell or to a 996, filed on Dec. 30, 1999, now abandoned, filed as Subject a replication decreasing amount of a GnRH agonist application No. PCT/DE98/01902 on Jul. 3, 1998. and/or GnRH antagonist and/or an erythropoietin agonist, and/or a thrombopoietin agonist, and/or a endothelin antago (30) Foreign Application Priority Data nist and/or a gonadotropin inhibiting hormone agonist is also provided. -

Necessity for LH in Selection and Continued Growth of the Bovine Dominant Follicle
159 5 REPRODUCTIONRESEARCH Necessity for LH in selection and continued growth of the bovine dominant follicle Victor E Gomez-León1,2,3, O J Ginther1,4, Rafael R Domingues1,4, José D Guimarães3 and Milo C Wiltbank2 1Eutheria Foundation, Cross Plains, Wisconsin, USA, 2Department of Dairy Science, University of Wisconsin- Madison (UW-Madison), Madison, Wisconsin, USA, 3Department of Veterinary Medicine, Universidade Federal de Viçosa, Viçosa, Brazil and 4Department of Pathobiological Science, School of Veterinary Medicine, UW-Madison, Madison, Wisconsin, USA Correspondence should be addressed to M C Wiltbank; Email: [email protected] Abstract Previous research demonstrated that acute treatment with GnRH antagonist, Acyline, allowed follicle growth until ~8.5 mm and no dominant follicle was selected. This study evaluated whether deficient LH was the underlying mechanism for Acyline effects by replacing LH action, using human chorionic gonadotropin (hCG), during Acyline treatment. Holstein heifers (n = 24) during first follicular wave were evaluated by ultrasound and randomized into one of three treatments: Control (saline treatments), Acyline (5 µg/ kg Acyline), or Acyline+hCG (Acyline plus 50 IU of hCG at start then 100 IU every 12 h). Pulses of LH were present in Control heifers (9 Pulses/10 h) but not during Acyline treatment. Data were normalized to the transition to diameter deviation (day 0; F1 ~7.5 mm). Diameter deviation of the largest (F1) and the second largest (F2) follicle was not observed in Acyline-treated heifers, whereas control heifers had decreased growth of F2 at F1 ~7.5 mm, indicating deviation. Selection of a single dominant follicle was restored by providing LH activity in Acyline+hCG heifers, as evidenced by F1 and F2 deviation, continued growth of F1, and elevated circulating estradiol. -

Kisspeptin Regulates Steroidogenesis and Spermiation in Anuran Amphibian
REPRODUCTIONRESEARCH Kisspeptin regulates steroidogenesis and spermiation in anuran amphibian Rosanna Chianese1, Vincenza Ciaramella2, Silvia Fasano1, Riccardo Pierantoni1 and Rosaria Meccariello3 1Dipartimento di Medicina Sperimentale sez ‘F. Bottazzi’, Università degli Studi della Campania ‘Luigi Vanvitelli’, Napoli, Italy, 2Dipartimento Medico-Chirurgico di Internistica Clinica e Sperimentale ‘F. Magrassi-L. Lanzara’, Università degli Studi della Campania ‘Luigi Vanvitelli’, Napoli, Italy and 3Dipartimento di Scienze Motorie e del Benessere, Università di Napoli Parthenope, Napoli, Italy Correspondence should be addressed to R Meccariello; Email: [email protected] Abstract Kisspeptin (Kp) system has a recognized role in the control of gonadotropic axis, at multiple levels. Recently, a major focus of research has been to assess any direct activity of this system on testis physiology. Using the amphibian anuran, Pelophylax esculentus, as animal model, we demonstrate – for the first time in non-mammalian vertebrate – that testis expresses both Kiss-1 and Gpr54 −6 proteins during the annual sexual cycle and that ex vivo 17B-estradiol (E2, 10 M) increases both proteins over control group. Since the interstitium is the main site of localization of both ligand and receptor, its possible involvement in the regulation of steroidogenesis has been evaluated by ex vivo treatment of testis pieces with increasing doses of Kp-10 (10−9–10−6 M). Treatments have been carried out in February – when a new wave of spermatogenesis occurs – and affect the expression of key enzymes of steroidogenesis inducing opposite effects on testosterone and estradiol intratesticular levels. Morphological analysis of Kp-treated testes reveals higher number of tubules with spermatozoa detached from Sertoli cells than control group and the expression of connexin 43, the main junctional protein in testis, is deeply affected by the treatment. -
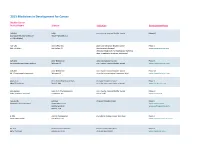
Adis R&D Insight
2015 Medicines in Development for Cancer Bladder Cancer Product Name Sponsor Indication Development Phase ABI-009 AADi non-muscle invasive bladder cancer Phase I/II (nanoparticle albumin-bound Pacific Palisades, CA mTOR inhibitor) ACP-196 Acerta Pharma platinum-refractory bladder cancer Phase II (Btk inhibitor) San Carlos, CA (combination therapy) www.acerta-pharma.com (see also head/neck, hematological, leukemia, lung, lymphoma, myeloma, pancreatic) ALT-801 Altor BioScience advanced bladder cancer, Phase II (immunotherapy fusion protein) Miramar, FL non-muscle invasive bladder cancer www.altorbioscience.com ALT-803 Altor BioScience non-muscle invasive bladder cancer Phase I/II (IL-15 superagonist complex) Miramar, FL (see also hematological, myeloma, skin) www.altorbioscience.com apatorsen OncoGenex Pharmaceuticals metastatic bladder cancer Phase II (Hsp27 inhibitor) Bothell, WA (see also lung, pancreatic, prostate) www.oncogenex.com apaziquone Spectrum Pharmaceuticals non-muscle invasive bladder cancer Phase III (DNA synthesis inhibitor) Henderson, NV (Fast Track) www.sppirx.com ASG-15ME Agensys relapsed bladder cancer Phase I (antibody drug conjugate) Santa Monica, CA www.agensys.com Seattle Genetics www.seattlegenetics.com Bothell, WA B-701 BioClin Therapeutics metastatic bladder cancer (2nd-line) Phase II (anti-FGFR3 mAb) San Ramon, CA www.bioclintherapeutics.com BC-819 BioCancell Therapeutics bladder cancer (2nd-line) Phase II (gene therapy) Jerusalem, Israel (see also pancreatic) www.biocancell.com Bladder Cancer Product Name Sponsor -

Reversible Downregulation of Hypothalamic-Pituitary-Gonadal Axis in the Stallion with a Third-Generation Gnrh Antagonist" (2015)
University of Kentucky UKnowledge Theses and Dissertations--Veterinary Science Veterinary Science 2015 REVERSIBLE DOWNREGULATION OF HYPOTHALAMIC- PITUITARY-GONADAL AXIS IN THE STALLION WITH A THIRD- GENERATION GNRH ANTAGONIST Gabriel Monteiro Davolli University of Kentucky, [email protected] Right click to open a feedback form in a new tab to let us know how this document benefits ou.y Recommended Citation Monteiro Davolli, Gabriel, "REVERSIBLE DOWNREGULATION OF HYPOTHALAMIC-PITUITARY-GONADAL AXIS IN THE STALLION WITH A THIRD-GENERATION GNRH ANTAGONIST" (2015). Theses and Dissertations--Veterinary Science. 22. https://uknowledge.uky.edu/gluck_etds/22 This Master's Thesis is brought to you for free and open access by the Veterinary Science at UKnowledge. It has been accepted for inclusion in Theses and Dissertations--Veterinary Science by an authorized administrator of UKnowledge. For more information, please contact [email protected]. STUDENT AGREEMENT: I represent that my thesis or dissertation and abstract are my original work. Proper attribution has been given to all outside sources. I understand that I am solely responsible for obtaining any needed copyright permissions. I have obtained needed written permission statement(s) from the owner(s) of each third-party copyrighted matter to be included in my work, allowing electronic distribution (if such use is not permitted by the fair use doctrine) which will be submitted to UKnowledge as Additional File. I hereby grant to The University of Kentucky and its agents the irrevocable, non-exclusive, and royalty-free license to archive and make accessible my work in whole or in part in all forms of media, now or hereafter known.