Malignant Transformation Within Benign Adnexal Skin Tumours
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Dermoscopic Features of Trichoadenoma
Dermatology Practical & Conceptual Broadening the List of Basal Cell Carcinoma Mimickers: Dermoscopic Features of Trichoadenoma Riccardo Pampena1, Stefania Borsari1, Simonetta Piana2, Caterina Longo1,3 1 Centro Oncologico ad Alta Tecnologia Diagnostica, Azienda Unità Sanitaria Locale - IRCCS di Reggio Emilia, Italy 2 Pathology Unit, Azienda Unità Sanitaria Locale - IRCCS di Reggio Emilia, Italy 3 Department of Dermatology, University of Modena and Reggio Emilia, Modena, Italy Key words: trichoadenoma, basal cell carcinoma, adnexal tumors, dermoscopy Citation: Pampena R, Borsari S, Piana S, Longo C. Broadening the list of basal cell carcinoma mimickers: dermoscopic features of trichoadenoma. Dermatol Pract Concept. 2019;9(2):160-161. DOI: https://doi.org/10.5826/dpc.0902a17 Accepted: January 10, 2019; Published: April 30, 2019 Copyright: ©2019 Pampena et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Funding: This research was supported by Italian Ministry of Health (Project Code: NET-2011-02347213). Competing interests: The authors have no conflicts of interest to disclose. Authorship: All authors have contributed significantly to this publication. Corresponding author: Riccardo Pampena, MD, Centro Oncologico ad Alta Tecnologia Diagnostica, Azienda Unità Sanitaria Locale – IRCCS, Viale Risorgimento 80, 42123, Reggio Emilia, Italy. Email: [email protected] Introduction Case Presentation A wide spectrum of skin tumors may mimic basal cell carci- Dermoscopic evaluation was performed with a contact polar- noma (BCC) on both clinical and dermoscopic appearance. ized dermatoscope (DermLite Foto, 3Gen LLC, Dana Point, Among these, adnexal skin neoplasms and in particular CA, USA) and showed a general BCC-like appearance. -

Microcystic Adnexal Carcinoma: Review of a Potential Diagnostic Pitfall and Management
Microcystic Adnexal Carcinoma: Review of a Potential Diagnostic Pitfall and Management Lana H. McKinley, DO; Stacey Seastrom, DO; Andrew J. Hanly, MD; Richard A. Miller, DO Practice Points Microcystic adnexal carcinoma (MAC) is a rare, locally aggressive, malignant cutaneous neoplasm with pilar and eccrine gland differentiation. Microcystic adnexal carcinoma should be considered in the differential diagnosis for slow-growing tumors of the head and neck. Initial misdiagnosis of MAC is possible, as the superficial histologic findings often mimic benign follicu- lar neoplasms. Mohs micrographic surgeCUTISry is the treatment of choice for MAC. Microcystic adnexal carcinoma (MAC) is an uncom- carcinoma with syringomatous features.1 Clinically, it mon, locally aggressive cutaneous neoplasm that presents as a slow-growing, asymptomatic lesion on usually presents as a slow-growing, asymptomatic the head or neck. Microcystic adnexal carcinoma has lesion on the head or neck. Microcystic adnexal a predilection for white individuals and has a slight carcinoma frequently is misdiagnosed due to female predominance.2 It frequently is misdiagnosed its histologic appearance on superficial biopsy due to its histologic appearance on superficial biopsy specimensDo mimicking other follicularNot neoplasms. specimens Copy mimicking other follicular neoplasms. Herein, we highlight a case in which a slow- growing lesion was initially diagnosed as a tricho- Case Report adenoma following superficial biopsy; however, A 90-year-old white woman presented with a lesion on after surgical excision the pathology revealed a the left side of the upper lip of 1 year’s duration. She locally aggressive MAC. denied pain, bleeding, pruritus, or history of a similar Cutis. 2014;93:162-165. -
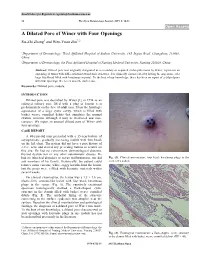
A Dilated Pore of Winer with Four Openings Ru-Zhi Zhang1 and Wen-Yuan Zhu*,2
Send Orders for Reprints to [email protected] 10 The Open Dermatology Journal, 2015, 9, 10-11 Open Access A Dilated Pore of Winer with Four Openings Ru-Zhi Zhang1 and Wen-Yuan Zhu*,2 1Department of Dermatology, Third Affiliated Hospital of Suzhou University, 185 Juqian Road, Changzhou, 213003, China 2Department of Dermatology, the First Affiliated Hospital of Nanjing Medical University, Nanjing 210029, China Abstract: Dilated pore was originally designated as a secondary or acquired trichoepithelioma by Winer, represents an appendageal tumor with differentiation towards hair structures. It is clinically characterized by having the appearance of a large blackhead filled with keratinous material. To the best of our knowledge, there has been no report of a dilated pore with four openings. We herein describe such a case. Keywords: Dilated pore, nodule. INTRODUCTION Dilated pore was described by Winer [1] in 1954 as an enlarged solitary pore filled with a plug of keratin seen predominately on the face of adult men. It has the histologic appearance of a large cystic cavity, which is filled with basket weave cornified debris that simulates the normal stratum corneum although it may be thickened and more compact. We report an unusual dilated pore of Winer with four openings. CASE REPORT A 68-year-old man presented with a 35-year-history of asymptomatic, gradually increasing nodule with four heads on the left chest. The patient did not have a past history of severe acne and denied any preceding trauma or scratch on this site. He had no concomitant dermatological diseases, thyroid dysfunction or any other autoimmune disease. -

Trichoadenoma of the Upper Lip Gian Paolo Bombeccari, Gianpaolo Guzzi, Umberto Mariani, Andrea Gianatti, Diego Ruffoni, Franco Santoro, Francesco Spadari
CASE REPORTS SCIENTIFIC ARTICLES Stomatologija, Baltic Dental and Maxillofacial Journal, 17: 102-4, 2015 Trichoadenoma of the upper lip Gian Paolo Bombeccari, Gianpaolo Guzzi, Umberto Mariani, Andrea Gianatti, Diego Ruffoni, Franco Santoro, Francesco Spadari SUMMARY Background. Trichoadenoma of Nikolowski, who describe the first cases in 1958, is a rare and benign tumor of the hair follicle. It is well-differentiated and slowly-growing. The clinical appearance of Trichoadenoma (TA) can be similar to basal cell carcinoma or epidermal cyst. Results. We describe a 44-year-old male who was referred for nodular lesion on the upper lip and a TA was diagnosed. Oral examination showed exophytic yellow mass located between mucous membrane of the upper lip and vestibular gingiva, 1.2 per 0.8 cm. Anamnestic data was non-contributory. An excisional biopsy of the lesion was performed. Microscopically, the lesion consisted of multiple keratinous cysts lined with stratified squamous epithelium and intermingled with solid islands of basaloid cells lying within sclerotic stroma. The pathological diagnosis was TA. The surgical wound healed uneventfully. Conclusion. Because the lesion is unique, it is uncertain how aggressive or indolent the tumor might be. Therefore, the microscopical analysis is mandatory. At the best of our knowledge, this is the second case of trichoadenoma of the lip. Keywords: lip lesion, oral nodule, lip tumor, oral tumor, oral follicular hamartoma. INTRODUCTION Trichoadenoma (TA) is a rare benign tumor of than trichofolliculoma and is more differentiated the hair follicle, which was first described in 1958 by than trichoepithelioma (4). It is often apparent a dif- Nikolowsky as “organoid follicular hamartoma” (1). -

Current Diagnosis and Treatment Options for Cutaneous Adnexal Neoplasms with Apocrine and Eccrine Differentiation
International Journal of Molecular Sciences Review Current Diagnosis and Treatment Options for Cutaneous Adnexal Neoplasms with Apocrine and Eccrine Differentiation Iga Płachta 1,2,† , Marcin Kleibert 1,2,† , Anna M. Czarnecka 1,* , Mateusz Spałek 1 , Anna Szumera-Cie´ckiewicz 3,4 and Piotr Rutkowski 1 1 Department of Soft Tissue/Bone Sarcoma and Melanoma, Maria Sklodowska-Curie National Research Institute of Oncology, 02-781 Warsaw, Poland; [email protected] (I.P.); [email protected] (M.K.); [email protected] (M.S.); [email protected] (P.R.) 2 Faculty of Medicine, Medical University of Warsaw, 02-091 Warsaw, Poland 3 Department of Pathology and Laboratory Diagnostics, Maria Sklodowska-Curie National Research Institute of Oncology, 02-781 Warsaw, Poland; [email protected] 4 Department of Diagnostic Hematology, Institute of Hematology and Transfusion Medicine, 00-791 Warsaw, Poland * Correspondence: [email protected] or [email protected] † Equally contributed to the work. Abstract: Adnexal tumors of the skin are a rare group of benign and malignant neoplasms that exhibit morphological differentiation toward one or more of the adnexal epithelium types present in normal skin. Tumors deriving from apocrine or eccrine glands are highly heterogeneous and represent various histological entities. Macroscopic and dermatoscopic features of these tumors are unspecific; therefore, a specialized pathological examination is required to correctly diagnose patients. Limited Citation: Płachta, I.; Kleibert, M.; treatment guidelines of adnexal tumor cases are available; thus, therapy is still challenging. Patients Czarnecka, A.M.; Spałek, M.; should be referred to high-volume skin cancer centers to receive an appropriate multidisciplinary Szumera-Cie´ckiewicz,A.; Rutkowski, treatment, affecting their outcome. -

Trichoblastic Carcinoma
SM Journal of Clinical Pathology Case Report © Azevedo C. et al. 2020 Trichoblastic Carcinoma: A Case Report and Literature Review of an Extremely Rare and Diagnostically Challenging Cutaneous Malignancy of the Hair Follicle Chelsea Azevedo*, Joseph Varney, Karina Leyva, Matthew White, Nicole DiTommaso, Alexandria Kim, Emily Alimia, Cameron Volpe, and Mohamed Aziz Department of Pathology- American University of the Caribbean, School of Medicine, USA Abstract Trichoblastic carcinoma (TBC) is an extremely rare cutaneous malignancy of the hair follicle, first described in the literature in 1962 as a “primary neoplasm of the hair matrix” (1). Besides its rarity, TBC shares many clinical and histologic characteristics with basal cell carcinoma (BCC), making the diagnosis of TBC difficult to make. Many authors have described TBC as a malignant transformation of a benign trichoblastoma (TB), but a universal understanding of the pathophysiological origin of TBC has not been established (2, 3). We present a case of this uncommon tumor with the hope that this report will add to the small repertoire of available research, and aid clinicians in diagnosis and management of this rare tumor. Keywords: Trichoblastic, Carcinoma, Cutaneous, Basal cell carcinoma, Stroma, Hair follicle neoplasm. Mitosis Abbreviations TBC, it is important to distinguish TBC from other skin tumors with similar clinical presentations such as TB and BCC as TBC is : Trichoblastic Carcinoma. : Basal Cell Carcinoma. TBC BCC typically aggressive and has a high propensity to metastasize and TB: Trichoblastoma. TBCS: Trichoblastic Carcinosarcoma Introduction recurThis [11]. case represents an extremely rare entity with very few Trichoblastic carcinoma (TBC) is a rare malignant skin published cases and reports available. -

Trichoadenoma in a Mature Cystic Teratoma: a Rare Finding P
Trichoadenoma In A Mature Cystic Teratoma: A Rare Finding P. Kaur, R. Bansal, M. Madan, Nutan Pathology Department, Giansagar Medical College and Hospital, Banur, Dist Patiala, Punjab, India Abstract trichoadenoma has also been described briefly. Skin adnexal tumors arising in dermoid cysts Keywords: of the ovary are exceedingly rare. We report a trichoadenoma arising in a dermoid cyst in trichoadenoma, mature cystic teratoma a 42-year-old female. The histopathology of Introduction hospital on day 5 after surgery. The left ovarian Mature teratomas, which are almost all cystic mass measured 8 x 6 x 3.5 cm. The outer surface (dermoid cysts), account for approximately was tan and smooth. Cut section was partly solid 25% of all ovarian tumors, and 30% of and partly cystic. Multiloculated cysts filled with benign ovarian tumors. They usually develop cheese-like material and hairs were seen. Slides in children or women of the reproductive age were prepared, stained with hematoxylin-eosin group(1). Histologically, they are composed of and seen under the light microscope. variable proportions of tissue originating from Sections from the cystic areas showed a lining the ectoderm, mesoderm, and endoderm. Cystic of keratinizing stratified squamous epithelium, cavities are lined by mature epidermis. Although including sebaceous glands, sweat glands and skin appendages and neural tissue are extremely hair. Neural tissue, respiratory and gastric common, there are only few case reports of skin epithelium, fat, bone and thyroid follicles were adnexal tumors arising in a mature teratoma. also identified. We report a case of ovarian teratoma with a One of the sections taken from the solid area trichoadenoma. -

Multiple Microcystic Adnexal Carcinomas
CONTINUING MEDICAL EDUCATION Multiple Microcystic Adnexal Carcinomas Robert N. Page, MD; Matthew C. Hanggi, MD; Roy King, MD; Paul B. Googe, MD GOAL To understand microcystic adnexal carcinomas (MACs) to better manage patients with the condition OBJECTIVES Upon completion of this activity, dermatologists and general practitioners should be able to: 1. Discuss the distribution of MACs. 2. Describe the histology of MACs. 3. Identify treatment options for MACs. CME Test on page 306. This article has been peer reviewed and approved Einstein College of Medicine is accredited by by Michael Fisher, MD, Professor of Medicine, the ACCME to provide continuing medical edu- Albert Einstein College of Medicine. Review date: cation for physicians. March 2007. Albert Einstein College of Medicine designates This activity has been planned and imple- this educational activity for a maximum of 1 AMA mented in accordance with the Essential Areas PRA Category 1 CreditTM. Physicians should only and Policies of the Accreditation Council for claim credit commensurate with the extent of their Continuing Medical Education through the participation in the activity. joint sponsorship of Albert Einstein College of This activity has been planned and produced in Medicine and Quadrant HealthCom, Inc. Albert accordance with ACCME Essentials. Drs. Page, Hanggi, King, and Googe report no conflict of interest. The authors report no discussion of off-label use. Dr. Fisher reports no conflict of interest. Microcystic adnexal carcinoma (MAC) is a rela- Case Report tively uncommon adnexal neoplasm that can An otherwise healthy 34-year-old black man presented demonstrate locally aggressive behavior; rare for evaluation of a progressive lesion on the left thigh instances of metastatic lesions have been that had been diagnosed as lichen simplex chronicus reported. -

ASCP. Cutaneous Adnexal Neoplasms: Classification and A
1355 Cutaneous Adnexal Neoplasms: Classification And A Practical Diagnostic Approach David S. Cassarino, MD, PhD, FASCP WEEKEND OF PATHOLOGY AMERICAN SOCIETY FOR CLINICAL PATHOLOGY 33 W Monroe Ste 1600 Chicago, IL 60603 Program Content and Disclosure The primary purpose of this activity is educational and the comments, opinions, and/or recommendations expressed by the faculty or authors are their own and not those of the ASCP. There may be, on occasion, changes in faculty and program content. In order to ensure balance, independence, objectivity, and scientific rigor in all its educational activities, and in accordance with ACCME Standards, the ASCP requires all individuals in positions to influence and/or control the content of ASCP CME activities to disclose whether they do or do not have any relevant financial relationships with proprietary entities producing health care goods or services that are discussed in the CME activities, with the exemption of non-profit or government organizations and non-health care related companies. These relationships are reviewed and any identified conflicts of interest are resolved prior to the activity. Faculty are asked to use generic names in any discussion of therapeutic options, to base patient care recommendations on scientific evidence, and to base information regarding commercial products/services on scientific methods generally accepted by the medical community. All ASCP CME activities are evaluated by participants for the presence of any commercial bias and this input is utilized for subsequent CME planning decisions. The individuals below have responded that they have no relevant financial relationships with commercial interests to disclose: Course Faculty: David S. -

Areclinicianssuccessful in Diagnosingcutaneousadnexaltumors? Aretrospective, Clinicopathologicalstudy
Turkish Journal of Medical Sciences Turk J Med Sci (2020) 50: 832-843 http://journals.tubitak.gov.tr/medical/ © TÜBİTAK Research Article doi:10.3906/sag-2002-126 Areclinicianssuccessful in diagnosingcutaneousadnexaltumors? aretrospective, clinicopathologicalstudy 1, 1 1 Melek ASLAN KAYIRAN *, Ayşe Serap KARADAĞ , Yasin KÜÇÜK , 2 1 1 Bengü ÇOBANOĞLU ŞİMŞEK , Vefa Aslı ERDEMİR , Necmettin AKDENİZ 1 Department of Dermatology, Göztepe Training and Research Hospital, İstanbul Medeniyet University, İstanbul, Turkey 2 Department of Pathology, Göztepe Training and Research Hospital, İstanbul Medeniyet University, İstanbul, Turkey Received: 15.02.2020 Accepted/Published Online: 11.04.2020 Final Version: 23.06.2020 Background/aim: Cutaneous adnexal tumors (CAT) are rare tumors originating from the adnexal epithelial parts of the skin. Due to its clinical and histopathological characteristics comparable with other diseases, clinicians and pathologists experience difficulties in its diagnosis. We aimed to reveal the clinical and histopathological characteristics of the retrospectively screened cases and to compare the prediagnoses and histopathological diagnoses of clinicians. Materials and methods: The data of the last 5 years were scanned and patients with histopathological diagnosis of CAT were included in the study. Results: A total of 65 patients, including 39 female and 26 male patients aged between 8 and 88, were included in the study. The female to male ratio was 1.5, and the mean age of the patients was 46.15 ± 21.8 years. The benign tumor rate was 95.4%, whereas the malignant tumor rate was 4.6%. 38.5% of the tumors were presenting sebaceous, 35.4% of them were presenting follicular, and 18.5% of them were presenting eccrine differentiation. -

Adnexal Tumours of the Skin J Clin Pathol: First Published As 10.1136/Jcp.44.7.543 on 1 July 1991
J Clin Pathol 199 1;44:543-548 543 Troublesome tumours 1: Adnexal tumours of the skin J Clin Pathol: first published as 10.1136/jcp.44.7.543 on 1 July 1991. Downloaded from D Cotton Introduction these are very unusual,6 and the confusion due Most adnexal tumours are benign and, if com- to the term "cylindroma" being used for a pletely excised, cause no further concern. It different, malignant, tumour of other sites may therefore be thought that there is little causes considerable difficulty. Again, duct dif- need for further subclassification. The major ferentiation is CEA positive, but the bulk of arguments for considering them further can tumour cells in all these tumours (poromas, be summarised as follows: (1) if you are not spiradenomas, and cylindromas) are CEA sure what it is, it may be something else; (2) negative. All of the above mentioned tumours clinical associations with specific subtypes will have features reminiscent of the sweat gland not become apparent if the lesions are never on electron microscopical examination and subtyped; and (3) there is academic and obses- they stain variably positive with middle sional satisfaction to be derived from weight cytokeratin antibodies such as PKKI meticulously identifying lesions as accurately and are negative for CAM5 2, S100, epithelial as possible. membrane antigen (EMA) and human milk Given these justifications I will comment on fat globule 1 (HMFG 1). what I consider to be useful and interesting Poroma, spiradenoma, and cylindroma are aspects of certain adnexal tumours. The first all derived from the outer cells of the duct and division is into tumours showing affinity with behave as benign "epitheliomas" or eccrine glands and those showing affinity with "basalomas" as these terms are variously used the pilosebaceous system. -

JMSCR Vol||08||Issue||02||Page 566-568||February 2020
JMSCR Vol||08||Issue||02||Page 566-568||February 2020 http://jmscr.igmpublication.org/home/ ISSN (e)-2347-176x ISSN (p) 2455-0450 DOI: https://dx.doi.org/10.18535/jmscr/v8i2.98 Dilated Pore of Winer Mimicking Trichofolliculoma Authors Dr J.Harshini1, Dr M.S.Srinivasan2 1Junior Resident, 2Head of the Department Department of Dermatology, Venereology and Leprosy, Chettinad Hospital and Research Institute, Kelambakkam *Corresponding Author Dr J. Harshini Junior resident, Department of Dermatology, Venereology and Leprosy, Chettinad Hospital and Research Institute, Kelambakkam Abstract Dilated pore of Winer which was originally designated as a secondary or acquired trichoepithelioma by Winer, represents an appendageal tumor with differentiation towards follicular structures. It is clinically characterised by having the appearance of a large comedone filled with keratinous material. To the best of our knowledge, there has been no report of dilated pore with a terminal hair in the centre. We herein describe such a case. Keywords: Dilated pore, Winer, Appendageal tumor, Trichoepithelioma. Introduction Case Report Dilated pore of Winer was first described by A 21 year old female presented with an Winer in 1954[1]. It is a commonly occurring asymptomatic slow growing swelling on her benign adnexal tumor of follicular neck for preceding six months. There was no differentiation[3][4]. It is commonly located on systemic symptoms and no history of similar head and neck, however dilated pore of Winer illness in the family. can also be found on the trunk of middle aged On examination a solitary skin coloured, shiny, and elderly individuals. These clinically present firm and non-tender papule was seen above the as an asymptomatic solitary enlarged pore with a right Clavicle [Figure 1].