February 2010 MONTHLY EDUCATIONAL CONFERENCE
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Systematized Verrucous Epidermal Nevus- a Case Report
Jebmh.com Case Report Systematized Verrucous Epidermal Nevus- A Case Report B.C. Sharathkumar1, N.S. Anitha2 1Professor and HOD, Department of Dermatology, Venereology and Leprosy, Kempegowda Institute of Medical Sciences Hospital and Research Centre, Bengaluru, Karnataka. 2Resident, Department of Dermatology, Venereology and Leprosy, Kempegowda Institute of Medical Sciences Hospital and Research Centre, Bengaluru, Karnataka. INTRODUCTION Veracious epidermal nevus (VEN) is a keratinocyte hematoma, usually present at Corresponding Author: birth or can develop later in life.1,2 It commonly involves trunk and limbs. Less Dr. N. S. Anitha, Resident, commonly, involved sites are head and neck; face is known to involve even more Department of Dermatology, 3,4,5 rarely. Clinical variants of VEN are zosteriform, linear, unilateral or systematized Venereology and Leprosy, 6 patterns with streaks and swirls. We report a case of girl who presented with Kempegowda Institute of Medical extensive VEN causing a lot of disfigurement. Epidermal nevi are hamartomas of Sciences Hospital and Research Centre, cutaneous structures arising from the embryonic ectoderm. The prevalence rate is Bengaluru- 560004, Karnataka. 1 in 1000, without predilection to race and sex.7,8 They are further divided into E-mail: [email protected] nonorganoid (Keratinocytic) types and organoid types (due to hyperplasia of DOI: 10.18410/jebmh/2019/637 adnexal structures such as sebaceous glands, sweat glands and hair follicles).8,9 Financial or Other Competing Interests: All epidermal nevi represents disorder of dermo-epidermal interactions and most None. cases are sporadic. Verrucous epidermal nevus is the most common type of keratinocyte nevus and derives its name from its keratotic and warty appearance. -

Atypical Compound Nevus Arising in Mature Cystic Ovarian Teratoma
J Cutan Pathol 2005: 32: 71–123 Copyright # Blackwell Munksgaard 2005 Blackwell Munksgaard. Printed in Denmark Journal of Cutaneous Pathology Abstracts of the Papers Presented at the 41st Annual Meeting of The American Society of Dermatopathology Westin Copley Place Boston, Massachusetts, USA October 14–17, 2004 These abstracts were presented in oral or poster format at the 41st Annual Meeting of The American Society of Dermatopathology on October 14–17, 2004. They are listed on the following pages in alphabetical order by the first author’s last name. 71 Abstracts IN SITU HYBRIDIZATION IS A VALUABLE DIAGNOSTIC A 37-year-old woman with diagnosis of Sjogren’s syndrome (SS) TOOL IN CUTANEOUS DEEP FUNGAL INFECTIONS presented with asymptomatic non-palpable purpura of the lower J.J. Abbott1, K.L. Hamacher2,A.G.Bridges2 and I. Ahmed1,2 extremities. Biopsy of a purpuric macule revealed a perivascular Departments of Laboratory Medicine and Pathology1 and and focally nodular lymphocytic infiltrate with large numbers of Dermatology2, plasma cells, seemingly around eccrine glands. There was no vascu- litis. The histologic findings in the skin were strikingly similar to those Mayo Clinic and Mayo Foundation, Rochester, MN, USA of salivary, parotid, and other ‘‘secretory’’ glands affected in SS. The cutaneous manifestations of SS highlighted in textbooks include Dimorphic fungal infections (histoplasmosis, blastomycosis, coccidiomy- xerosis, annular erythema, small-vessel vasculitis, and pigmented cosis, and cryptococcosis) can occur in immunocompromised and purpura. This case illustrates that purpura in skin of patients with healthy individuals. Cutaneous involvement is often secondary and SS may be caused by a peri-eccrine plasma-rich infiltrate. -

第32回日本皮膚病理組織学会学術大会 診断投票結果 口演 1 Drug Eruption 13, うち Erythema Multif
第32回日本皮膚病理組織学会学術大会 診断投票結果 口演 1 Drug eruption 13, うち erythema multiforme 1, Interface dermatitis 1, GVHD type 1 Cutaneous reaction due to CCR4 3, うち Dysplastic epidermal hyperplasia 2, Adverse reaction 1 Erythema multiforme 3 PLEVA 1 Vacuolar type interface dermatitis 1 口演 2 Syringofibroadenoma 15, うち + amyloid 1 Syringofibroadenoma with BCC 5 Basal cell carcinoma 4, うち Pinkus type of BCC with syringofibroadenoma 2 口演 3 Darier disease 5 Hailey-Hailey disease 4 Pemphigus 3, うち Pemphigus Vegetans 1, Neonatal pemphigus 1 Grover's disease 4 Epidermal nevus 5, うち Acantholytic (dyskeratotic) epidermal nevus 4, Linear epidermal nevus 1 口演 4 Hydradenoma 13, うち Clear cell hidradenoma 12, Nodular hidradenoma 1 Sebaceous adenoma 1 Trichilemmoma 1 Metastatic tumor 8, うち ~ renal carcinoma6, ~ Clear cell carcinoma 2 口演 5 Apocrine carcinoma 3, うち ~with pagetoid spreading 2 Ectopic breast carcinoma(invasive ductal type)with pagetoid phenomenon 2 Extramammary Paget's disease 12, うち Paget carcinoma 3, ~ with Apocrine adenoma 2, ~ with Tubular adenoma 1, Invasive ~ 1, +Skin metastasis 1, With syringoma 1, with Microcystic Adnexal Carcinoma 1 Syringomatous carcinoma 2, うち ~with paget phenomenon 1 Tubular adenocartinoma 1 Tubular (apocrine) adenoma 2 Syringoma 1 口演 6 Dermatofibroma 10, うち Lipidized ~ 3, Hemosiderotic deep cellular ~ 2, Xanthomatous ~ 1, ~ Histiocytoid variant 1 Fibous histiocytoma 8, うち Atypical ~ 3, Malignant ~ 2, Aneurismal ~ 2 Undifferentiated pleomorphic sarcoma 2 Progressive nodular histiocytosis 1 Squamous -

An Institutional Experience
Original Research Article Skin Adnexal Tumors- An Institutional Experience 1 2* 3 4 5 6 Rekha M Haravi , Roopa K N , Priya Patil , Rujuta Datar , Meena N Jadhav , Shreekant K Kittur 1,5Associate Professor, 2Post Graduate Student, 3,4Assistant Professor, 6Professor & HOD, Department of Pathology, Belgaum Institute of Medical Sciences Dr B R Ambedkar Road, Belagavi, Karnataka – 590001, INDIA. Email: [email protected] Abstract Background: Skin adnexal tumors are a wide spectrum of benign and malignant tumors that differentiate towards one or more adnexal structures found in normal skin. The adnexal structures of skin are the hair follicles, sebaceous glands, eccrine and apocrine sweat glands. These skin adnexal tumors are often difficult to diagnose clinically. This retrospective study was undertaken to know the various histomorphological patterns of skin adnexal tumors at our institution and to determine the incidence among the genders and age groups along with the site distribution. Materials and methods: A total of 40 specimens received and diagnosed as skin adnexal tumors in the department of Pathology at Belgaum Institute of Medical Sciences, Belagavi for a period of 6 years from January 2014 to December 2019 were taken for the study. Histopathological slides prepared from tissue blocks retrieved from departmental archives were reviewed and classified according to the WHO classification 2017. Results: Out of the total 40 samples, benign tumors were 36 (90%) and malignant were 4 (10%). Largest group was the benign tumors of apocrine and eccrine differentiation (47.5%) followed by benign tumors of hair follicle differentiation (40%). Malignant tumors of sebaceous differentiation were 5%, malignant tumors of eccrine and apocrine differentiation were 2.5% and malignant hair follicle differentiation tumors were 2.5% of the total. -

The Tamilnadu Dr. M.G.R. Medical University Chennai, Tamil Nadu
CLINICO-PATHOLOGICAL STUDY OF SKIN SURFACE EPIDERMAL AND APPENDAGEAL TUMOURS Dissertation Submitted in partial fulfillment of university regulations for M.D. DEGREE IN DERMATOLOGY, VENEREOLOGY AND LEPROSY BRANCH XII – A THE TAMILNADU DR. M.G.R. MEDICAL UNIVERSITY CHENNAI, TAMIL NADU SEPTEMBER 2006 CERTIFICATE This is to certify that this Dissertation entitled “CLINICO-PATHOLOGICAL STUDY OF SKIN SURFACE EPIDERMAL AND APPENDAGEAL TUMOURS” is a bonafide work done by DR.G.BALAJI, Postgraduate student of Department of Dermatology, Leprosy and Institute of STD, Madras Medical College and Government General Hospital, Chennai – 3 for the award of Degree of M.D.( Dermatology, Venereology and Leprosy ) Branch XII – A during the academic year of 2003-2006. This work has not previously formed in the basis for the award of any degree or diploma. Prof. Dr. B. Parveen, MD., DD., Professor & Head, Dept. of Dermatology and Leprosy, Madras Medical College & Govt. General Hospital, Chennai – 3. Prof. Dr. Kalavathy Ponniraivan, MD., The Dean Madras Medical College & Govt. General Hospital, Chennai – 3. SPECIAL ACKNOWLEDGEMENT I sincerely thank Prof. Dr. Kalavathy Ponniraivan, MD., Dean, Madras Medical College & Govt. General Hospital, Chennai – 3, for granting me permission to use the resources of this institution for my study. ACKNOWLEDGEMENT I sincerely thank Prof. B.Parveen MD.,DD, Professor and Head of Department of Dermatology for her invaluable guidance and encouragement for the successful completion of this study. I express my heart felt gratitude to Dr.N.Gomathy MD.,DD, former Head of department of Dermatology who was instrumental in the initiation of this project, giving constant guidance throughout my work. -

Pdf 344.65 K
Differential Diagnosis of Basal Cell Carcinoma and Benign Tumors of Hair Follicles Using CD34 RESEARCH COMMUNICATION Differential Diagnosis of Basal Cell Carcinoma and Benign Tumors of Cutaneous Appendages Originating from Hair Follicles by Using CD34 Demet Sengul1, Ilker Sengul2*, Muzeyyen Hesna Astarci3, Huseyin Ustun3, Gamze Mocan4 Abstract Background and Aims: Differential diagnosis of the group of benign trichoblastomas, trichofolliculomas, trichoadenomas and trichoepitheliomas, and basal cell carcinomas (BCCs) is troublesome for the clinician as well as the pathologist, especially when only small biopsy specimens are available. Here we investigated whether CD34 expression might be of assistance. Methods: Thirty benign tumors of cutaneous appendages originating from hair follicles (BTCOHF) and 30 BCCs were retrieved from our archives and immunohistochemically stained. CD 34 expression was graded from [0] to [2+] and compared among the groups and subgroups. Results: There was no significant difference between the degree of expression between [0] and [1+] and [0] and [2+] for each group. However, [1+] and [2+] immunopositivity of BTCOHFs was significantly stronger than in BCCs (p= 0.014). Conclusions: CD34 may contribute to differential diagnosis of skin lesions. Keywords: Basal cell cancer - hair follicle lesions - CD 34 immunohistochemistry Asian Pacific J Cancer Prev, 11, 1615-1619 Introduction in 1958. TAs occur as a nodular lesion usually on the face and buttocks (Rahbari et al., 1977, Swaroop et al., 2008) Ackerman et al classified benign tumors of cutaneous and have a variant of verrucous TA mimicing seboreic appendages originating from hair follicle (BTCOHF)’s keratosis. using eight textbooks of dermatopathology in 2001 as Trichoepithelioma (TE) is a benign skin tumor with germ tumors of hair follicle and hamartomas, infindubular follicular differentiation determined in the classification and isthmic tumors, tumors of external layer, tumors of WHO as the synonym of TB (Cotton, 1991). -

Rippled-Pattern Sebaceoma: a Report of a Lesion on the Back with a Review of the Literature
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by University of Fukui Repository Rippled-pattern sebaceoma: A report of a lesion on the back with a review of the literature Takahiro Kiyohara, M.D., Masanobu Kumakiri, M.D., Hiroaki Kuwahara, M.D., Atsuko Saitoh, M.D., and Shinichi Ansai, M.D. Department of Dermatology (T.K., M.K.), University of Fukui, Fukui; Division of Plastic Surgery (H.K.), Obihiro-Kousei General Hospital, Obihiro: Sapporo Institute for Dermatopathology (S.A.), Sapporo, Japan Address correspondence and reprint requests to: Takahiro Kiyohara, M.D. Department of Dermatology, University of Fukui 23-3 Shimoaizuki, Matsuoka-cho, Yoshida-gun, Fukui 910-1193, Japan Tel: +81 776 61 3111 Fax: +81 776 61 8112 e-mail: kiyo @ fmsrsa.fukui-med.ac.jp Abstract A 68-year-old Japanese man presented with a tumor that had been present for 5 to 6 years on the right back. Physical examination revealed a dome-shaped, 12x13-mm, dark red tumor. The tumor was excised with a 2 to 3-mm margin. The patient has remained free of disease during 77-months of follow-up. Microscopic examination revealed a bulb-like tumor in the dermis, contiguous with the overlying epidermis. It was composed of small, monomorphous, cigar-shaped basaloid cells in linear, parallel rows, resembling the palisading of nuclei of Verocay bodies, and presenting a rippled-pattern. There were scattered cells showing sebaceous differentiation with vacuolated cytoplasm and scalloped nuclei. There were tiny, duct-like spaces. The tumor revealed characteristics of rippled-pattern sebaceoma. -

Nevus Sebaceus with Syringocystadenoma
UC Davis Dermatology Online Journal Title Nevus sebaceus with syringocystadenoma papilliferum, prurigo nodularis, apocrine cystadenoma, basaloid follicular proliferation, and sebaceoma: case report and review of nevus sebaceus-associated conditions Permalink https://escholarship.org/uc/item/85k968bk Journal Dermatology Online Journal, 26(2) Authors Basu, Pallavi Erickson, Christof P Calame, Antoanella et al. Publication Date 2020 DOI 10.5070/D3262047411 License https://creativecommons.org/licenses/by-nc-nd/4.0/ 4.0 Peer reviewed eScholarship.org Powered by the California Digital Library University of California Volume 26 Number 2| February 2020| Dermatology Online Journal || Case Report 26(2):5 Nevus sebaceus with syringocystadenoma papilliferum, prurigo nodularis, apocrine cystadenoma, basaloid follicular proliferation, and sebaceoma: case report and review of nevus sebaceus-associated conditions Pallavi Basu1 MPH, Christof P Erickson2 MD, Antoanella Calame2 MD, and Philip R Cohen3,4 MD Affiliations: 1School of Medicine, University of California San Diego, La Jolla, California, USA, 2Compass Dermatopathology, San Diego, California, USA, 3San Diego Family Dermatology, National City, California, USA, 4Touro California College of Osteopathic Medicine, Vallejo, California, USA Corresponding Authors: Pallavi Basu, MPH, 8528 Via Mallorca, Apartment G, La Jolla, CA 92037, Tel: 818-917-1786, Email: [email protected]; Philip R. Cohen MD, 10991 Twinleaf Court, San Diego, CA 92131, Email: [email protected] with nevus sebaceus who not only developed Abstract syringocystadenoma papilliferum but also prurigo Nevus sebaceus is a benign skin hamartoma of nodularis in the inferior portion of her lesion is congenital onset that grows during puberty, and in described. Complete excision of the residual nevus adulthood can develop secondary benign and sebaceus also revealed three concurrent additional malignant neoplasms. -

2016 Essentials of Dermatopathology Slide Library Handout Book
2016 Essentials of Dermatopathology Slide Library Handout Book April 8-10, 2016 JW Marriott Houston Downtown Houston, TX USA CASE #01 -- SLIDE #01 Diagnosis: Nodular fasciitis Case Summary: 12 year old male with a rapidly growing temple mass. Present for 4 weeks. Nodular fasciitis is a self-limited pseudosarcomatous proliferation that may cause clinical alarm due to its rapid growth. It is most common in young adults but occurs across a wide age range. This lesion is typically 3-5 cm and composed of bland fibroblasts and myofibroblasts without significant cytologic atypia arranged in a loose storiform pattern with areas of extravasated red blood cells. Mitoses may be numerous, but atypical mitotic figures are absent. Nodular fasciitis is a benign process, and recurrence is very rare (1%). Recent work has shown that the MYH9-USP6 gene fusion is present in approximately 90% of cases, and molecular techniques to show USP6 gene rearrangement may be a helpful ancillary tool in difficult cases or on small biopsy samples. Weiss SW, Goldblum JR. Enzinger and Weiss’s Soft Tissue Tumors, 5th edition. Mosby Elsevier. 2008. Erickson-Johnson MR, Chou MM, Evers BR, Roth CW, Seys AR, Jin L, Ye Y, Lau AW, Wang X, Oliveira AM. Nodular fasciitis: a novel model of transient neoplasia induced by MYH9-USP6 gene fusion. Lab Invest. 2011 Oct;91(10):1427-33. Amary MF, Ye H, Berisha F, Tirabosco R, Presneau N, Flanagan AM. Detection of USP6 gene rearrangement in nodular fasciitis: an important diagnostic tool. Virchows Arch. 2013 Jul;463(1):97-8. CONTRIBUTED BY KAREN FRITCHIE, MD 1 CASE #02 -- SLIDE #02 Diagnosis: Cellular fibrous histiocytoma Case Summary: 12 year old female with wrist mass. -
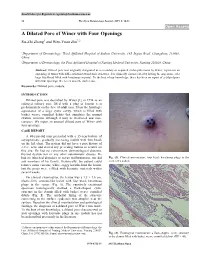
A Dilated Pore of Winer with Four Openings Ru-Zhi Zhang1 and Wen-Yuan Zhu*,2
Send Orders for Reprints to [email protected] 10 The Open Dermatology Journal, 2015, 9, 10-11 Open Access A Dilated Pore of Winer with Four Openings Ru-Zhi Zhang1 and Wen-Yuan Zhu*,2 1Department of Dermatology, Third Affiliated Hospital of Suzhou University, 185 Juqian Road, Changzhou, 213003, China 2Department of Dermatology, the First Affiliated Hospital of Nanjing Medical University, Nanjing 210029, China Abstract: Dilated pore was originally designated as a secondary or acquired trichoepithelioma by Winer, represents an appendageal tumor with differentiation towards hair structures. It is clinically characterized by having the appearance of a large blackhead filled with keratinous material. To the best of our knowledge, there has been no report of a dilated pore with four openings. We herein describe such a case. Keywords: Dilated pore, nodule. INTRODUCTION Dilated pore was described by Winer [1] in 1954 as an enlarged solitary pore filled with a plug of keratin seen predominately on the face of adult men. It has the histologic appearance of a large cystic cavity, which is filled with basket weave cornified debris that simulates the normal stratum corneum although it may be thickened and more compact. We report an unusual dilated pore of Winer with four openings. CASE REPORT A 68-year-old man presented with a 35-year-history of asymptomatic, gradually increasing nodule with four heads on the left chest. The patient did not have a past history of severe acne and denied any preceding trauma or scratch on this site. He had no concomitant dermatological diseases, thyroid dysfunction or any other autoimmune disease. -

Epidermal Nevus Syndrome with Development of a Mandibular
Vol. 90 No. 1 July 2000 ORAL SURGERY ORAL MEDICINE ORAL PATHOLOGY ORAL AND MAXILLOFACIAL PATHOLOGY Editor: Alan R. Gould Epidermal nevus syndrome with development of a mandibular ameloblastoma Euterpe Bazopoulou-Kyrkanidou, DDS, MD, MS, Dr Dent,a Constantin Alexandridis, DDS, MS, Dr Dent,b Konstantinos I. Tosios, DDS, Dr Dent,c Stela Sotiriadou, DDS, Dr Dent,d and Angelos P. Angelopoulos, DDS, PhD,e Athens, Greece UNIVERSITY OF ATHENS Epidermal nevus syndrome (ENS) is a hamartoneoplastic syndrome characterized by the association of epidermal nevi with abnormalities in other organ systems. We report a 32-year-old woman with ENS that, in addition to cutaneous mani- festations, showed red plaques on the maxillary and mandibular labial alveolar mucosa and a papillomatous lesion of the midline posterior hard palate. Radiographic examination of the jaws was noncontributory. Approximately 5 years later, a follic- ular ameloblastoma developed in the mandible. The tumor showed duct-like cystic spaces, continuity with the overlying epithelium, and globular myxomatous areas in the connective tissue. The palatal lesion was diagnosed as papilloma, whereas the maxillary plaques showed nonspecific mucositis. The association of ameloblastoma with ENS is discussed. This is the second case of ENS associated with ameloblastoma reported in the medical literature. (Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2000;90:64-70) Epidermal nevus syndrome (ENS) is a hamartoneoplastic epidermal nevus syndrome has been used for lesions syndrome1 characterized -

CCI 221.01 Medical Accession Standards 12 July 2019
CCI 221.01 Medical Accession Standards 12 July 2019 Appendix A Disqualifying Medical and Dental Conditions Table of Contents Condition Page I. Head and Neck…………………………………………………………………………… 5 II. Mouth, Nose, Larynx and Trachea…………………………………………………… 6 III. Dental Disorders…………………………………………………………..…………… 7 IV. Eyes and Vision………………………………………………………………………... 8 V. Ears and Hearing…………………………………………………………...…………… 11 VI. Cardiovascular Disorders………………………………………………….…………… 12 VII. Pulmonary Disorders……………………………………………………..…………… 15 VIII. Gastrointestinal and Hepatobiliary Disorders…………………………...………… 17 IX. Endocrine and Metabolic Disorders…………………………………………………… 20 X. Hematological Disorders…………………………………………………..…………… 22 XI. Renal and Urologic Disorders…………………………………………….…………… 23 XII. Gynecological Disorders and Breast Disease……………………………………… 26 XIII. Musculoskeletal and Rheumatologic Disorders………………………..…………… 28 XIV. Skin Disorders………………………………………………………….……………… 35 XV. Infectious Diseases…………………………………………………………………… 38 XVI. Immunologic Disorders……………………………………………………………… 39 XVII. Neoplastic Disorders…………………………………………………...…………… 41 XVIII. Neurologic and Muscle Disorders…………………………………….…………… 42 XIX. Mental Disorders………………………………………………………..…………… 45 XX. Substance Use and Addictive Behaviors…………………………………………… 48 XXI. Miscellaneous…………………………………………………………...…………… 49 4 CCI 221.01 Medical Accession Standards 12 July 2019 Condition Disqualification for Appointment I. Head and Neck A. Deformities of the 1. Deformity of the skull, face, or mandible which is a skull manifestation of an