Distinct Brain Structure and Behavior Related to ADHD and Conduct Disorder Traits
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

HPP and Behavioral Update
Provider Directory Health Partnership Plan And Behavioral Health 2013 Plan Year (Updated June 15, 2013) County of Volusia Group No. 2081 Health Partnership Plan Contact Information County of Volusia (Group 2081) Claims Information & Plan Inquiries MedSave USA, Inc. (formerly FSAI) 3035 Lakeland Hills Boulevard (855) 224-5173 Lakeland, FL 33805 (516) 622-1705 Claims Fax: (863) 291-5010 Insurance-Benefit Offices Personnel Division (386) 736-5951 Website : https://www.medsaveadmin.com Benefits Section (386) 740-5137 Provider Network Website: http://volusia.org/personnel/benefits or For questions regarding physician providers you have http://enn.co.volusia.fl.us/ several networks at your disposal. Please call any of the networks listed below. Medical Pre-certification Requirements • Florida Memorial Health Network 24-HOUR NURSE HELP LINE (386) 231-4398 (877) 582-7061 (888) 839-7430 Website: http://www.fmhn.org KePRO Pre-certification (888) 522-7742 You must pre-certify with KePRO the following • Complete Health Network for Osceola, procedures before services are rendered by your Lake, Seminole or Orange provider in order to avoid a penalty . Counties Only (800) 741-4869 In-patient Hospital Stay – call 7 days prior to admis- (407) 741-4869 sion or the next working day after an emergency admis- sion. Website: http://www.fhhsonline.com - excluding chiropractic Outpatient surgical and medical services which require pre-certification • Volusia Health Network (Refer to your insurance card or call KePRO 7 (386) 425-4VHN (4846) days prior to date -

Acomb County, Michigan, Naturalization Index
LAST NAME FIRST NAME MIDDLE NAME FIRST PAPER SECOND PAPER Cain John V52, P1903 V52, P1903 Cain Joseph James Thomas V41, P1030 V41, P1030 Cain Willfred Douglas V19, P2195 Cairns Mary Colville V91, P8334 Cairns Thomas James V91, P8335 Calagna Michela V92, P8542 Calcagno Vita Gentile V69, P3851 Calcaterra Lottie Bernice V88, P7678 Calcheff Peter V11, P273 Calgrove Charles Robert V7, P66 Calhoun Michael Raymond V89, P7934 Calibeo Dora Altera V75, P4634 Calisto Maria Munzia V22, P2558 Calisto Maria Nunzia V67, P3574 Callaghan Sybil Ivy V78, P5152 Callebaut Frans Livinus V78, P5304 Callebaut Maria Amandina V78, P5276 Calleja Robert John V86, P7167 Callens Irma V64, P3220 Callewaert Arsena Theresa V70, P3945 Callewaert Conrad V14, P76 Callewaert Gentill V33, P33 V33, P33 Callewaert Julia V47, P1410 Callewaert Marie V21, P2410 Callewaert Marie V60, P2740 V60, P2740 Callewaert Mary V35, P651 Callewaert Maurits V17, P1968 Callewaert Maurits V45, P1235 V45, P1235 Monday, July 22, 2002 Page 96 of 807 LAST NAME FIRST NAME MIDDLE NAME FIRST PAPER SECOND PAPER Callewaert Sidonia V64, P3213 Callihan John V3, P195 Callihan Patrick V3, P197 Calmi Crescentino V84, P6753 Calonne Nicolle V. V80, P5789 Caltagirone Caterina V86, P7151 Caltoum Antoine V87, P7252 Caltoum Hanna Jerjis V91, P8430 Caltoum John V91, P8430 Calvert Henry Redvers V23, P2924 Calvert John Norman Whitfield Canad V8, P70 Calvert Martha Fraser V21, P2423 Calvert Martha Fraser V60, P2735 V60, P2735 Camaj Shaqe V94, P9136 Camaj Shasha V94, P9136 Camaj Teresza V93, P9069 Cambridge Clara Winifred Irene V66, P3468 Camfield Jeffrey George V32, P21 V32, P21 Camilleri Charles (Carmelo) V64, P3246 Camilleri Mary V90, P8115 Camilleri Paul Emanuel V90, P8116 Cammarata William (Vito) V50, P1710 Campagne Lawrence V63, P3155 Campau Frank John V9, P77 Campau William J. -
Order Copies of Records by Calling (517
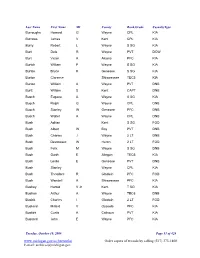
Last Name First Name MI County Rank/Grade CasualtyType Burroughs Howard G Wayne CPL KIA Burrows James V Kent CPL KIA Burry Robert L Wayne S SG KIA Burt Dale R Wayne PVT DOW Burt Victor A Alcona PFC KIA Burtch William P Wayne S SG KIA Burton Bruce K Genesee S SG KIA Burton Clarence Shiawassee TEC5 KIA Burton William A Wayne PVT DNB Burtt William S Kent CAPT DNB Busch Eugene A Wayne S SG KIA Busch Ralph G Wayne CPL DNB Busch Stanley W Genesee PFC DNB Busch Walter A Wayne CPL DNB Bush Adrian Kent S SG FOD Bush Albert W Bay PVT DNB Bush Charles J Wayne 2 LT DNB Bush Devereaux W Huron 2 LT FOD Bush Felix M Wayne S SG DNB Bush Garth E Allegan TEC5 KIA Bush Leslie E Genesee PVT DNB Bush Stanley Wayne CPL KIA Bush Theodore R Gladwin PFC FOD Bush Wendell A Shiawassee PFC KIA Bushey Harold V Jr Kent T SG KIA Bushon Arthur A Wayne TEC5 DNB Busick Charles I Gladwin 2 LT FOD Buskard Millard V Osceola PFC KIA Buskirk Curtis A Calhoun PVT KIA Bussard John E Wayne PFC KIA Tuesday, October 10, 2006 Page 51 of 429 www.michigan.gov/archivesofmi Order copies of records by calling (517) 373-1408 E-mail: [email protected] Last Name First Name MI County Rank/Grade CasualtyType Bustraan Corwin Kent PVT KIA Buswink Joseph G Wayne PFC DOW Butcher Eustace Wayne SGT KIA Butera Carl Wayne S SG KIA Buteyn William E Ottawa PFC KIA Butka Dimitri Wayne PVT KIA Butkowski Roman Wayne PFC KIA Butler Arnold G Oakland PFC KIA Butler Harry J Wayne PVT KIA Butler Howard E Wayne TEC5 DNB Butterfield Carl N Bay PVT KIA Butterfield Wilbur B Wayne CAPT DNB Butters William T -

Iall Pointes Primp!7 Candidates of the I
-,. - -, _.~~.....-..,....~-_.-----...--...._.-_-- ...--------------------------------------~-~-~~----~_.~-----~._---_._.._-~ -"--' ~.~ -, -'- ~ ~ . , - , '~ l. ., ' All the News of All the Pointes Every Thursday Morning rosse.Pointe ews Conlplete ~TelVSCoverage of All the Pointes Home of the News VOL. 29-No. 20 Enl;;;d~-;s.;cond ci~~s-r.i~ii.ral GROS ..Sr:-P.O.-1N-TE--M .....I.C... H.. IGAN MAY ~.oo Per Year --- -'t~h::e-'p:::o':'sl~~O£fic_e__ a_l_D_e_l_rO_it._M __iC_hl_g_an a;; , __' 16, 1968 lOe Per Copy 48 Pages - Two Sections - Section One ---_._-----_._----------------------------------------------_._---_ .._--- r-U-E-A-D-I-~I-N-ES-' Concert Raises Music Scholarship Funds IAll Pointes Primp!7 Candidates of the I. l'In Race for \VEt:K For Celebratwn of Six Offices As Compiled by the Proposed Charter Amend. _G_TOSS_e PO_inte _News.....J Michigan Week ment W~uld ~ncreasel Employes PensIons as ; Thursday. 1Ilay 9 Urged by Trustees NEGOTIATORS for the De. trait Free Press and the Detroit Mayors' Exchange Day Set For Monday Is Highlight News continued to bargain with I of Annual Observance; Visiting Officials to Seven candidates h a v e the Pressmen's Union, one of i riled intentions to seek four on strike, in an attempt to i Be Feted at Luncheon at GPYC municipal offices in the end the 175,day shutdown of the, ----------- Shores, at the regular ViI. cily's daily papers, U,S. District i . All five Grosse Pointes are being mowed, curried, lage election to be held on Court Judge Damon Keith said'i pohshed, and otherwise spruced up, as are hundreds of Tuesday, May 21, it was a temporary restraining order I other c?mmunities throughout the state, ill preparation disclosed by VI'llage Super- b a I' r in g the inl~rvention o{ for .their annual obs ervance 0 f M'ICh'Igan 'V. -

May 7, 2000 Hometownnewspapers
lomeTOwn COMMUNICATIONS N B T W O ft K Wcstlano <8)b0mrer Your hometown newspaper serving Westland for 35 years Sunday, May 7, 2000 hometownnewspapers. net 75c Volume 35 Number 97 Westland, Michigan 00000 Hometown Communications Network™ THE WEEK AHEAD MONDAY Council business: West- land City Council study sessions on the city bud get will begin 5:30p.m. May 8 at City Hall, on Ford near Carlson. An 8:30 p.m. session on the clerk consultant contract is planned. Budget ses sions continue May 10 and May 17. School board: The Wayne- Westland Board of Edu Pray with me: Clasped hands cation will meet 7p.m. express the deep emotion that May 8at the board office, was prevalent among wor on Marquette between , STAJT PBOTt* BY KvrTWtV TArtJNCtK shippers who gathered Newburgh and Wayne Praise God: Susan May, a former drug addict who found religion after failed rehab attempts, Thursday afternoon in front roads in Westland. spreads her arms wide during an emotional moment in prayer at Westland City Hall, of City Hall. TUESDAY Mayor speaks: Westland Prayer prompts calm protest Mayor Robert Thomas will speak to the Westland National Day of Prayer at Westland City Hall Views: included a group of atheists this year. Mem Henry Mor Chamber of Commerce. bers of American Athei sts Inc. oppose the gan, Michi The business luncheon use ofjzovernment property to endorse reli gan state begins 11:30 a.m. May 9 gion. The protest was peaceful. director of at Joy Manor, on Joy east BY KURT KUBAN of .government and religion,- came to the Ameri of Middlebelt in West- STAFF WRITER protest the event, at which partici can Athe land. -

U.S. Department of Justice Federal Bureau of Investigation Washington, D.C. 20535 August 24, 2020 MR. JOHN GREENEWALD JR. SUITE
U.S. Department of Justice Federal Bureau of Investigation Washington, D.C. 20535 August 24, 2020 MR. JOHN GREENEWALD JR. SUITE 1203 27305 WEST LIVE OAK ROAD CASTAIC, CA 91384-4520 FOIPA Request No.: 1374338-000 Subject: List of FBI Pre-Processed Files/Database Dear Mr. Greenewald: This is in response to your Freedom of Information/Privacy Acts (FOIPA) request. The FBI has completed its search for records responsive to your request. Please see the paragraphs below for relevant information specific to your request as well as the enclosed FBI FOIPA Addendum for standard responses applicable to all requests. Material consisting of 192 pages has been reviewed pursuant to Title 5, U.S. Code § 552/552a, and this material is being released to you in its entirety with no excisions of information. Please refer to the enclosed FBI FOIPA Addendum for additional standard responses applicable to your request. “Part 1” of the Addendum includes standard responses that apply to all requests. “Part 2” includes additional standard responses that apply to all requests for records about yourself or any third party individuals. “Part 3” includes general information about FBI records that you may find useful. Also enclosed is our Explanation of Exemptions. For questions regarding our determinations, visit the www.fbi.gov/foia website under “Contact Us.” The FOIPA Request number listed above has been assigned to your request. Please use this number in all correspondence concerning your request. If you are not satisfied with the Federal Bureau of Investigation’s determination in response to this request, you may administratively appeal by writing to the Director, Office of Information Policy (OIP), United States Department of Justice, 441 G Street, NW, 6th Floor, Washington, D.C. -

Congressional Record-Senate. April 25
900 CONGRESSIONAL RECORD-SENATE. APRIL 25, Mr. ROOKER. I am not aware that it did, and if it did it did not the few days needed to secure a suitable location. We have taken affect it enough to make it successful. In my own district, when I care of hundreds of such people; we can take care of hundreds and was hundreds of miles away, I was elected by a majority of thousands thousands more; and we are willing to do so. We are entirely will against a man who came out in a secret circular and when I was not ing that these people should come; we want them to come; but we aware that I had any contestant. I desire to say that it was on that do not want them to come in such a way that the hands of our benev account that the vote of last year was different from the vote of for olence ancl philanthropy will be taxed beyond the possibility of pro mer years. But I am not to be driven by interrogatories of gentle viding for them. men from the charge I make upon you, that so far from being the Mr. HOOKER. Will the gentleman allow me to have read in re true friend of the colored man yQu have enacted in Northern States, ply to what he has just said a paragraph from one of the papers pub where you manumitted him, more onerous laws against him than lished in his State Y were ever enacted in the State of Mississippi. -

Annual Report 2008-2009 MOUNT SAINT MICHAEL, 4300 Murdock Avenue, Bronx, N
Volume XII, No. V Annual Report 2008-2009 MOUNT SAINT MICHAEL, 4300 Murdock Avenue, Bronx, N. Y October, 2009 ANNUAL REPORT President’s Message 2008-2009 HOT OFF THE PRESSES A MESSAGE FROM ANTHONY MISERANDINO, PH.D. Dear Alumni and Friends of the Mount, The Annual Report details the triumphs of the efforts from every corner of the Mount universe. The generosity of every member of our community never fails to impress and amaze me – thank you for supporting our boys! On behalf of the many families who have been hard hit by the recent grim economic and financial crisis, I say “thank you.” Without the generosity documented here, many of our students would not have had the opportunity to benefit from a Mount education and the considerable leveraging it creates for their college acceptance and scholarships (over $20 million for the Class of 2009). The MOUNT is thriving like never before! I am as grateful as I am enthusiastic about the tremendous support we have received from our extraordinarily faithful alumni and friends. Our recent induction ceremony of the third cohort of “Legends of the Mount” demonstrates the great achievement of spirit and resources generated to realize our mission of serving first generation students who often are marginalized by society. The mission and purpose of the MOUNT is as vibrant today as in the past: to provide a quality education and a Catholic community which nourishes the maturity and faith of all our members. This Annual Report is powerful testimony to the enthusiastic embrace of this mission that has served the young men of the MOUNT so well over our 83- year history. -

New York Public Service Commission
To: New York Public Service Commission Let’s build on New York’s solar success. I urge the PSC to approve the New York State Energy Research and Development Authority’s (NYSERDA) recent petition to extend the NY-Sun Initiative through 2023. Signed by 10,418 people: Name Postcode Address Raina Russo 11509 123 Bayside Drive Josh Nelson 20009 2637 16th Street John Baldwin 10016 223 East 28th St., New York, NY Josh Romano 10001 276 5th Ave Marisa Bartolucci 10001 252 7th Avenue, Apt 9N Murshed Zaheed 94105 101 Market Street James Mendelsohn 10011 350 West 21st Street, #3 Michael Bondoc 10009 11 avenue D Apt 12 Chung Wan 10011 26 W 17th Street Susan mohr 10007 100 CHURCH ST, nyc Harvey Lippman 10010 6 Peter Cooper Road steffani aarons 10003 200 Park Avenue South Juergen Bamberger 10002 568 Grand Street Saadique Abader 10001 22 Gideon Road Newfields Estate Bettina Stammen 10011 125 W. 12th St. #1C NYC Penelope Crabtree 10007 105 Duane Street, Apt 16E John Houshmand 13788 476 svr rd Hobart ny john o'sullivan 10003 55 e 7th st Katherine Slawinski 10003-3850 321 East 17 Street Richard P Faust 10007-3607 105 Duane St Ronald Reed 12534 21 prospect ave Jay Kidd 10011 37 W. 12th St., #2E, New York, NY michael d schaible 10011 8 west 13 st, nyc Sean Mahony 10010 531 East 20th Street Claudia Marx 10024 210 W. 90th Street Candice Myers 10011 42 W. 13th St., Apt. 3B V Brandt 10011 28 W. 10th St. 4R Name Postcode Address Prudence Brown 10025 380 Riverside Drive #5T Etan Harwayne- 10009 270 First Ave Gidansky Daniel Esakoff 10026 130 Malcolm X Blvd Leah Jacobs 10003 E. -

A Guide to Belfast and the Counties of Down & Antrim
Belfast adjacent coimtks. 'ju^- >*^^7^^- ^aL>w^>^( GUIDE TO BELFAST. ELFAST-Scal ROAD MAP OF THE ENVIRONS OF BELFAST-Scale 4 miles to an inch • ^ U '^ BELFAST, 1902. A Guide to Belfast AND THE COUNTIES OF DOWN & ANTRIM. PREPARED FOR THE MEETING OF THE BRITISH ASSOCIATION BY THE BELFAST NATURALISTS' FIELD CLUB. 'W JGelfaet: M'CAW, STEVENSON & OKK, LIMITED, THE MNENHAI.L PRESS. 1902. — PREFACE. 'hen the Brilisli Association met at Belfast in 1S74, a (luide was prepared for the occasion by the IkMfast NaturaHsts' Field Club, describing and illustrating the history, trade, agriculture, geology, botany, zoology, and archaeology of Belfast and the adjacent counties of Down and Antrim. This work was the first of the "Handbooks" which are now con- sidered an indispensable adjunct to the Association's meetings. The 1874 "Guide" long remained a standard work of reference on the district, and lately it has gone out of print. The present " Guide " has the same origin as its pre- decessor, and follows the same general lines. It has been written by members of the Belfast Field Club, with the co-operation, in certain subjects, of fellow-workers outside the Club, whose assistance is gratefully acknowledged : of Dr. R. F. Scharff, A. R. Nichols, G. H. Carpenter, and J. N. Halbert, all of the National Museum in Dublin, in the department of Zoology ; and of H. J. Seymour, of H.M. Geological Survey, in the department of Geology. The authorship of the various sections is as follows : Belfast, John Vinycomb and Alec Wilson ; Geology, Phillips J. St. J. ; Botany, Henry Hanna, R. -

Geburtsdaten II
Geburtsdaten II Aiuppa, Joseph 01.12.1907 Aivanhov, Omraam 31.01.1900 Akhurst, Daphne 22.04.1903 Geburtsjahrgänge 1900-1924 Akurgal, Ekrem 30.03.1911 Alascia, Vinve 14.01.1914 Aarons, Ruth H. 11.06.1910 Albach-Retty, Wolf 28.05.1906 Aba, Marta 25.06.1900 Albert, Abraham A. 09.11.1905 Abakarow, Rabadan 07.11.1917 Albert, Eddie 22.04.1908 Abakumow, Viktor 24.04.1908 Albertazzi, Giorgio 20.08.1923 Abbagnano, Nicola 15.07.1901 Albertsson, Bertil 01.09.1921 Abbandando, Frank 11.07.1910 Albertz, Heinrich 22.01.1915 Abe, Sada 28.05.1905 Albini, Franco 17.10.1905 Abegglen, Max 11.04.1902 Albrecht, Egon 19.05.1918 Abel, Rudolf 11.07.1903 Albrecht, Karl 20.02.1920 Abendroth, Wolfgang 02.05.1906 Albrecht, Theodor 28.03.1922 Abplanalp, Robert 04.04.1922 Albritton, David 13.04.1913 Abramovitz, Max 23.05.1908 Albus, Emmmy 13.12.1911 Abrashkin, Raymond 09.03.1911 Alder, Kurt 10.07.1902 Abrassimow, Pjotr A. 16.05.1912 Alderson, Samuel 21.10.1914 Abs, Hermann 15.10.1901 Aldrich, Robert 09.08.1918 Abshagen, Robert 12.01.1911 Alex, Hus 01.04.1916 Abzug, Bella 24.07.1920 Alexander, Alger 12.09.1900 Accardo, Anthony J. 28.04.1906 Alfrink, Bernard 05.07.1900 Ackerman, Forrest 24.11.1916 Alfvén, Hannes 30.05.1908 Acking, Carl-Axel 08.03.1910 Allais, Émile 25.02.1912 Ackva, Wolf 30.07.1911 Allais, Maurice 31.05.1911 Adair, Paul N. 18.06.1915 Allen, Harry 05.11.1911 Adam, Alfred 04.04.1908 Allen, Terry 08.06.1924 Adam, Henri-G. -

The Animated Movie Guide
THE ANIMATED MOVIE GUIDE Jerry Beck Contributing Writers Martin Goodman Andrew Leal W. R. Miller Fred Patten An A Cappella Book Library of Congress Cataloging-in-Publication Data Beck, Jerry. The animated movie guide / Jerry Beck.— 1st ed. p. cm. “An A Cappella book.” Includes index. ISBN 1-55652-591-5 1. Animated films—Catalogs. I. Title. NC1765.B367 2005 016.79143’75—dc22 2005008629 Front cover design: Leslie Cabarga Interior design: Rattray Design All images courtesy of Cartoon Research Inc. Front cover images (clockwise from top left): Photograph from the motion picture Shrek ™ & © 2001 DreamWorks L.L.C. and PDI, reprinted with permission by DreamWorks Animation; Photograph from the motion picture Ghost in the Shell 2 ™ & © 2004 DreamWorks L.L.C. and PDI, reprinted with permission by DreamWorks Animation; Mutant Aliens © Bill Plympton; Gulliver’s Travels. Back cover images (left to right): Johnny the Giant Killer, Gulliver’s Travels, The Snow Queen © 2005 by Jerry Beck All rights reserved First edition Published by A Cappella Books An Imprint of Chicago Review Press, Incorporated 814 North Franklin Street Chicago, Illinois 60610 ISBN 1-55652-591-5 Printed in the United States of America 5 4 3 2 1 For Marea Contents Acknowledgments vii Introduction ix About the Author and Contributors’ Biographies xiii Chronological List of Animated Features xv Alphabetical Entries 1 Appendix 1: Limited Release Animated Features 325 Appendix 2: Top 60 Animated Features Never Theatrically Released in the United States 327 Appendix 3: Top 20 Live-Action Films Featuring Great Animation 333 Index 335 Acknowledgments his book would not be as complete, as accurate, or as fun without the help of my ded- icated friends and enthusiastic colleagues.