Enlightening Butterfly Conservation Efforts
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

This File Was Created by Scanning the Printed
J Insect Behav DOl 10.1007/sI0905-011-9289-1 Evidence for Mate Guarding Behavior in the Taylor's Checkerspot Butterfly Victoria J. Bennett· Winston P. Smith· Matthew G. Betts Revised: 29 July 20 II/Accepted: 9 August 20II ��. Springer Seicnee+Business Media, LLC 2011 Abstract Discerning the intricacies of mating systems in butterflies can be difficult, particularly when multiple mating strategies are employed and are cryptic and not exclusive. We observed the behavior and habitat use of 113 male Taylor's checkerspot butterflies (Euphydryas editha taylori). We confinned that two distinct mating strategies were exhibited; patrolling and perching. These strategies varied temporally in relation to the protandrous mating system employed. Among perching males, we recorded high site fidelity and aggressive defense of small «5 m2) territories. This telTitoriality was not clearly a function of classic or non-classic resource defense (i.e., host plants or landscape), but rather appeared to constitute guarding of female pupae (virgin females). This discrete behavior is previously undocumented for this species and has rarely been observed in butterflies. Keywords Euphydryas editha taylori . mating systems · pre-copulatory mate guarding· protandry· sexual selection Introduction Inn'asexual selection is the most conunonly observed and well-documented sexual selection process exhibited by butterfly species (Andersson 1994; Rutowski 1997; Wiklund 2003). Competition between males for sexually receptive females has led to the evolution of a large variety of mating systems in this taxon (Rutowski 1991). Among these mating systems, mate acquisition strategies, such as mate locating, V J. Bennett (18) . M. G. Betts Department of Forest Ecosystems and Society, Oregon State University. -

Origins of Six Species of Butterflies Migrating Through Northeastern
diversity Article Origins of Six Species of Butterflies Migrating through Northeastern Mexico: New Insights from Stable Isotope (δ2H) Analyses and a Call for Documenting Butterfly Migrations Keith A. Hobson 1,2,*, Jackson W. Kusack 2 and Blanca X. Mora-Alvarez 2 1 Environment and Climate Change Canada, 11 Innovation Blvd., Saskatoon, SK S7N 0H3, Canada 2 Department of Biology, University of Western Ontario, Ontario, ON N6A 5B7, Canada; [email protected] (J.W.K.); [email protected] (B.X.M.-A.) * Correspondence: [email protected] Abstract: Determining migratory connectivity within and among diverse taxa is crucial to their conservation. Insect migrations involve millions of individuals and are often spectacular. However, in general, virtually nothing is known about their structure. With anthropogenically induced global change, we risk losing most of these migrations before they are even described. We used stable hydrogen isotope (δ2H) measurements of wings of seven species of butterflies (Libytheana carinenta, Danaus gilippus, Phoebis sennae, Asterocampa leilia, Euptoieta claudia, Euptoieta hegesia, and Zerene cesonia) salvaged as roadkill when migrating in fall through a narrow bottleneck in northeast Mexico. These data were used to depict the probabilistic origins in North America of six species, excluding the largely local E. hegesia. We determined evidence for long-distance migration in four species (L. carinenta, E. claudia, D. glippus, Z. cesonia) and present evidence for panmixia (Z. cesonia), chain (Libytheana Citation: Hobson, K.A.; Kusack, J.W.; Mora-Alvarez, B.X. Origins of Six carinenta), and leapfrog (Danaus gilippus) migrations in three species. Our investigation underlines Species of Butterflies Migrating the utility of the stable isotope approach to quickly establish migratory origins and connectivity in through Northeastern Mexico: New butterflies and other insect taxa, especially if they can be sampled at migratory bottlenecks. -

The Radiation of Satyrini Butterflies (Nymphalidae: Satyrinae): A
Zoological Journal of the Linnean Society, 2011, 161, 64–87. With 8 figures The radiation of Satyrini butterflies (Nymphalidae: Satyrinae): a challenge for phylogenetic methods CARLOS PEÑA1,2*, SÖREN NYLIN1 and NIKLAS WAHLBERG1,3 1Department of Zoology, Stockholm University, 106 91 Stockholm, Sweden 2Museo de Historia Natural, Universidad Nacional Mayor de San Marcos, Av. Arenales 1256, Apartado 14-0434, Lima-14, Peru 3Laboratory of Genetics, Department of Biology, University of Turku, 20014 Turku, Finland Received 24 February 2009; accepted for publication 1 September 2009 We have inferred the most comprehensive phylogenetic hypothesis to date of butterflies in the tribe Satyrini. In order to obtain a hypothesis of relationships, we used maximum parsimony and model-based methods with 4435 bp of DNA sequences from mitochondrial and nuclear genes for 179 taxa (130 genera and eight out-groups). We estimated dates of origin and diversification for major clades, and performed a biogeographic analysis using a dispersal–vicariance framework, in order to infer a scenario of the biogeographical history of the group. We found long-branch taxa that affected the accuracy of all three methods. Moreover, different methods produced incongruent phylogenies. We found that Satyrini appeared around 42 Mya in either the Neotropical or the Eastern Palaearctic, Oriental, and/or Indo-Australian regions, and underwent a quick radiation between 32 and 24 Mya, during which time most of its component subtribes originated. Several factors might have been important for the diversification of Satyrini: the ability to feed on grasses; early habitat shift into open, non-forest habitats; and geographic bridges, which permitted dispersal over marine barriers, enabling the geographic expansions of ancestors to new environ- ments that provided opportunities for geographic differentiation, and diversification. -
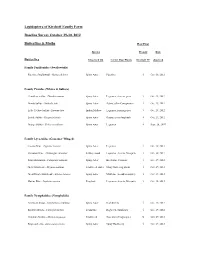
Butterflies and Moths List
Lepidoptera of Kirchoff Family Farm Baseline Survey October 29-30, 2012 Butterflies & Moths Host Plant Species Present Date Butterflies Observed On Larval Host Plants Kirchoff FF observed Family Papilionidae (Swallowtails) Pipevine Swallowtail - Battus philenor Spiny Aster Pipevine √ Oct. 30, 2012 Family Pieridae (Whites & Sulfurs) Cloudless Sulfur - Phoebis sennae Spiny Aster Legumes, clovers, peas √ Oct. 29, 2012 Dainty Sulfur - Nathalis iole Spiny Aster Asters, other Com;posites √ Oct. 29, 2012 Little Yellow Sulfur - Eurema lisa Indian Mallow Legumes, partridge pea √ Oct. 29, 2012 Lyside Sulfur - Kirgonia lyside Spiny Aster Guayacan or Soapbush √ Oct. 29, 2012 Orange Sulfur - Colias eurytheme Spiny Aster Legumes √ Sept. 26, 2007 Family Lycaenidae (Gossamer Winged) Cassius Blue - Leptotes cassius Spiny Aster Legumes √ Oct. 30, 2012 Ceraunus Blue - Hemiargus ceraunus Kidney wood Legumes- Acacia, Mesquite √ Oct. 30, 2012 Fatal Metalmark - Calephelis nemesis Spiny Aster Baccharis, Clematis √ Oct. 29, 2012 Gray Hairstreak - Strymon melinus Frostweed, Aster Many flowering plants √ Oct. 29, 2012 Great Purple Hairstreak - Atlides halesus Spiny Aster Mistletoe (in oak/mesquite) √ Oct. 29, 2012 Marine Blue - Leptotes marina Frogfruit Legumes- Acacia, Mesquite √ Oct. 30, 2012 Family Nymphalidae (Nymphalids) American Snout - Libytheana carinenta Spiny Aster Hackberries √ Oct. 29, 2012 Bordered Patch - Chlosyne lacinia Zexmenia Ragweed, Sunflower √ Oct. 29, 2012 Common Mestra - Mestra amymone Frostweed Noseburn (Tragia spp.) X Oct. 29, 2012 Empress Leila - Asterocampa leilia Spiny Aster Spiny Hackberry √ Oct. 29, 2012 Gulf Fritillary - Agraulis vanillae Spiny Aster Passion Vine X Oct. 29, 2012 Hackberry Emperor - Asterocampa celtis spiny Hackberry Hackberries √ Oct. 29, 2012 Painted Lady - Vanessa cardui Pink Smartweed Mallows & Thistles √ Oct. 29, 2012 Pearl Crescent - Phycoides tharos Spiny Aster Asters √ Oct. -

Butterflies in the Verde Valley
Butterflies in the Verde Valley 1 2 17 1. Mormon Fritillary, Speyeria mormonia 2. Empress Leilia, Asterocampa leilia 3. Fiery Skipper, Hylephila phyleus 4. Greenish-Blue Lycaenid, Plebejus saepiolus 16 4 18 Female on top, male below 3 5. Pipevine Swallowtail, Battus philenor 6. American Snout Butterfly, Libytheana carinenta 7. Cloudless Sulpher, Phoebis sennae 14 15 Female with patterned wing, male solid 5 8. Two-tailed Swallowtail, Papilio multicaudata 9. Variegated Fritillary, Euptoieta claudia 13 12 10. Hoary Comma, Polygonia gracilis 6 11. California Sister, Adelpha bredowii Eurema nicippe 8 12. Sleepy Orange Sulphur, 11 Two males 10 13. Alfalfa Sulfpur, Colias euretheme Two males, one female (pale) 14. Painted Lady, Vanessa cardui 15. Pine White, Neophasia menapia 9 7 16. Viceroy, Limenitus archippus 17. Queen, Danaus gilippus 18. Black Swallowtail, Papilio polyxenes Butterflies Butterflies are an amazing group of insects, and their delicate structure, flight, and colors, brighten our day. The butterflies in this display represent 6 related families of Lepidoptera (scaled wings) which we know as butterflies. All of these occur in northern Arizona. The best place to look for butterflies is often in moist creek beds and in areas where flowers are blooming. The mouth is composed of two long flexible straws that are connected. When they feed/drink they unroll the mouth parts (called a proboscis) and suck in nectar or other liquids. Although the shapes and colors differs, their basic structure is similar. All have four wings, six legs, an abdomen, thorax, head, and antenna. Butterflies have complete metamorphosis, with four separate stages: (1)egg, (2)larva, (3)chrysalis, and (4)adult. -

Final Lower Rio Grande Valley and Santa Ana National Wildlife
Final Lower Rio Grande Valley and Santa Ana National Wildlife Refuges Comprehensive Conservation Plan September 1997 (Reprint March 1999) U.S. Fish and Wildlife Service U.S. Department of the Interior Cover Artwork by Brian Cobble Table of Contents VISION........................................................................................................................................... 5 Executive Summary................................................................................................................... 6 1.0 Introduction and Regional Setting................................................................................. 8 1.1 LRGV Challenges............................................................................................... 8 2.0 Planning Perspectives and Considerations................................................................ 9 2.1 National Wildlife Refuge System ................................................................... 9 2.2 The Service & Ecosystem Management ...................................................... 9 2.3 Refuge Complex and Management Districts........................................... 10 2.4 Laguna Atascosa NWR -- A Partner with LRGV NWR............................ 10 2.5 Planning Perspectives.................................................................................... 10 2.6 The Issues.......................................................................................................... 11 2.7 The Need for Action........................................................................................ -

And Mission Blue Butterfly Populations Found at Milagra Ridge and the Mission Blue Butterfly Population at Marin Headlands Are Managed by the GGNRA
San Bruno Elfin Butterfly (Callophrys mossii bayensis) and Mission Blue Butterfly (Icaricia icarioides missionensis) 5-Year Review: Summary and Evaluation Photo by Patrick Kobernus: Adult male mission blue butterfly. Sacramento Fish and Wildlife Field Office U.S. Fish and Wildlife Service Sacramento, California February 2010 5-YEAR REVIEW San Bruno Elfin Butterfly (Callophrys mossii bayensis) and Mission blue butterfly (Icaricia icarioides missionensis) I. GENERAL INFORMATION Purpose of 5-Year Reviews: The U.S. Fish and Wildlife Service (Service) is required by section 4(c)(2) of the Endangered Species Act (Act) to conduct a status review of each listed species at least once every 5 years. The purpose of a 5-year review is to evaluate whether or not the species’ status has changed since it was listed (or since the most recent 5-year review). Based on the 5-year review, we recommend whether the species should be removed from the list of endangered and threatened species, be changed in status from endangered to threatened, or be changed in status from threatened to endangered. Our original listing of a species as endangered or threatened is based on the existence of threats attributable to one or more of the five threat factors described in section 4(a)(1) of the Act, and we must consider these same five factors in any subsequent consideration of reclassification or delisting of a species. In the 5-year review, we consider the best available scientific and commercial data on the species, and focus on new information available since the species was listed or last reviewed. -

Papilio (New Series) # 25 2016 Issn 2372-9449
PAPILIO (NEW SERIES) # 25 2016 ISSN 2372-9449 ERNEST J. OSLAR, 1858-1944: HIS TRAVEL AND COLLECTION ITINERARY, AND HIS BUTTERFLIES by James A. Scott, Ph.D. in entomology University of California Berkeley, 1972 (e-mail: [email protected]) Abstract. Ernest John Oslar collected more than 50,000 butterflies and moths and other insects and sold them to many taxonomists and museums throughout the world. This paper attempts to determine his travels in America to collect those specimens, by using data from labeled specimens (most in his remaining collection but some from published papers) plus information from correspondence etc. and a few small field diaries preserved by his descendants. The butterfly specimens and their localities/dates in his collection in the C. P. Gillette Museum (Colorado State University, Fort Collins, Colorado) are detailed. This information will help determine the possible collection locations of Oslar specimens that lack accurate collection data. Many more biographical details of Oslar are revealed, and the 26 insects named for Oslar are detailed. Introduction The last collection of Ernest J. Oslar, ~2159 papered butterfly specimens and several moths, was found in the C. P. Gillette Museum, Colorado State University, Fort Collins, Colorado by Paul A. Opler, providing the opportunity to study his travels and collections. Scott & Fisher (2014) documented specimens sent by Ernest J. Oslar of about 100 Argynnis (Speyeria) nokomis nokomis Edwards labeled from the San Juan Mts. and Hall Valley of Colorado, which were collected by Wilmatte Cockerell at Beulah New Mexico, and documented Oslar’s specimens of Oeneis alberta oslari Skinner labeled from Deer Creek Canyon, [Jefferson County] Colorado, September 25, 1909, which were collected in South Park, Park Co. -

Do Butterflies Use “Hearing Aids”? Investigating the Structure and Function of Inflated Wing Veins in Nymphalidae
Do butterflies use “hearing aids”? Investigating the structure and function of inflated wing veins in Nymphalidae by Penghui (Carrie) Sun A thesis submitted to the Faculty of Graduate and Postdoctoral Affairs in partial fulfillment of the requirements for the degree of Master of Biology in Biology Carleton University Ottawa, Ontario © 2018 Penghui (Carrie) Sun Abstract Many butterfly species within the subfamily Satyrinae (Nymphalidae) have been informally reported to possess a conspicuous “inflated” or “swollen” subcostal vein on each forewing. However, the function and taxonomic diversity of these structures is unknown. This thesis comprises both experimental and comparative approaches to test hypotheses on the function and evolution of these inflated veins. A laser vibrometry study showed that ears in the common wood nymph, Cercyonis pegala, are tuned to sounds between 1-5 kHz and the inflated subcostal vein enhances sensitivity to these sounds. A comparative study showed that all species with inflated veins possess ears, but not all species with ears possess inflated veins. Further, inflated veins were better developed in smaller butterflies. This thesis provides the first evidence for the function of inflated wing veins in butterflies and supports the hypothesis that they function as aids to low frequency hearing. ii Acknowledgements I thank my supervisor Dr. Jayne Yack for the continued guidance and support, throughout my academic program and in beginning my career, as well as an inspired and newfound appreciation I never knew I could have for insects. I thank my committee members Dr. Jeff Dawson and Dr. Charles-Antoine Darveau for their guidance, advice, and support. I thank Dr. -

Grasshoppers and Butterflies of the Quitobaquito Management Area, Organ Pipe Cactus National Monument, Arizona
1 2 COOPERATIVE NATIONAL PARK RESOURCES STUDIES UNIT UNIVERSITY OF ARIZONA 125 Biological Sciences (East) Bldg. 43 Tucson, Arizona 85721 R. Roy Johnson, Unit Leader National Park Senior Research Scientist TECHNICAL REPORT NO. 21 GRASSHOPPERS AND BUTTERFLIES OF THE QUITOBAQUITO MANAGEMENT AREA, ORGAN PIPE CACTUS NATIONAL MONUMENT, ARIZONA Kenneth J. Kingsley and Richard A. Bailowitz July 1987 NATIONAL PARK SERVICE/UNIVERSITY OF ARIZONA Contract No. 8100-3-0356 CONTRIBUTION NUMBER CPSU/UA 055/01 3 4 5 TABLE OF CONTENTS INTRODUCTION .......................................................................................................................... 1 DESCRIPTION OF THE AREA ................................................................................................... 2 METHODS ..................................................................................................................................... 4 DISCUSSION AND RESULTS .................................................................................................... 5 SPECIES ACCOUNTS ..................................................................................................... 8 Grasshoppers .........................................................................................................8 Butterflies ............................................................................................................11 LITERATURE CITED ................................................................................................................ 22 ACKNOWLEDGEMENTS......................................................................................................... -

Territorial Behavior of the Red Admiral Butterfly, Vanessa Atalanta (L.) (Lepidoptera: Nymphalidae) Royce Justin Bitzer Iowa State University
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1995 Territorial behavior of the Red Admiral Butterfly, Vanessa atalanta (L.) (Lepidoptera: Nymphalidae) Royce Justin Bitzer Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Ecology and Evolutionary Biology Commons, Entomology Commons, Environmental Sciences Commons, and the Zoology Commons Recommended Citation Bitzer, Royce Justin, "Territorial behavior of the Red Admiral Butterfly, Vanessa atalanta (L.) (Lepidoptera: Nymphalidae) " (1995). Retrospective Theses and Dissertations. 10881. https://lib.dr.iastate.edu/rtd/10881 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. INFORMATION TO USERS This manuscript has been reproduced from the miaofilm master. UMI films the text directly from the original or copy submitted. Thus, some thesis and dissertation copies are in typewriter face, while others may be from any type of conq)uter printer. The quality of this reproductioii is dependrat upon the quality of the copy submitted. Broken or indistinct print, colored or poor quality illustrations and photographs, print bleedthrough, substandard Tnarginc and inqiroper alignment can adversety affect reproduction. In the unlikely event that the author did not send UMI a complete manuscript and there are missing pages, these will be noted. Also, if unauthorized copyright material had to be removed, a note win indicate the deletion. -

Evidence for Mate Guarding Behavior in the Taylor's Checkerspot Butterfly
J Insect Behav (2012) 25:183–196 DOI 10.1007/s10905-011-9289-1 Evidence for Mate Guarding Behavior in the Taylor’s Checkerspot Butterfly Victoria J. Bennett & Winston P. Smith & Matthew G. Betts Revised: 29 July 2011 /Accepted: 9 August 2011 / Published online: 19 August 2011 # Springer Science+Business Media, LLC 2011 Abstract Discerning the intricacies of mating systems in butterflies can be difficult, particularly when multiple mating strategies are employed and are cryptic and not exclusive. We observed the behavior and habitat use of 113 male Taylor’s checkerspot butterflies (Euphydryas editha taylori). We confirmed that two distinct mating strategies were exhibited; patrolling and perching. These strategies varied temporally in relation to the protandrous mating system employed. Among perching males, we recorded high site fidelity and aggressive defense of small (<5 m2) territories. This territoriality was not clearly a function of classic or non-classic resource defense (i.e., host plants or landscape), but rather appeared to constitute guarding of female pupae (virgin females). This discrete behavior is previously undocumented for this species and has rarely been observed in butterflies. Keywords Euphydryas editha taylori . mating systems . pre-copulatory mate guarding . protandry . sexual selection Introduction Intrasexual selection is the most commonly observed and well-documented sexual selection process exhibited by butterfly species (Andersson 1994; Rutowski 1997; Wiklund 2003). Competition between males for sexually receptive females has led to the evolution of a large variety of mating systems in this taxon (Rutowski 1991). Among these mating systems, mate acquisition strategies, such as mate locating, V. J. Bennett (*) : M. G. Betts Department of Forest Ecosystems and Society, Oregon State University, Richardson Hall, Corvallis, OR 97331, USA e-mail: [email protected] W.