United States Patent Office Patented June 6, 1961
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Access to Aryl Mellitic Acid Esters Through a Surprising Oxidative Esterification Reaction Margarita R
CORE Metadata, citation and similar papers at core.ac.uk Provided by Digital Repository @ Iowa State University Chemistry Publications Chemistry 2014 Access to Aryl Mellitic Acid Esters through a Surprising Oxidative Esterification Reaction Margarita R. Geraskina Iowa State University, [email protected] Mark James Juetten Iowa State University, [email protected] Arthur Winter Iowa State University, [email protected] Follow this and additional works at: http://lib.dr.iastate.edu/chem_pubs Part of the Materials Chemistry Commons, Other Chemistry Commons, and the Physical Chemistry Commons The ompc lete bibliographic information for this item can be found at http://lib.dr.iastate.edu/ chem_pubs/146. For information on how to cite this item, please visit http://lib.dr.iastate.edu/ howtocite.html. This Article is brought to you for free and open access by the Chemistry at Iowa State University Digital Repository. It has been accepted for inclusion in Chemistry Publications by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. Access to Aryl Mellitic Acid Esters through a Surprising Oxidative Esterification Reaction Abstract A serendipitously discovered oxidative esterification reaction of cyclohexane hexacarboxylic acid with phosphorus pentachloride and phenols provides one-pot access to previously unknown aryl mellitic acid esters. The er action features a solvent-free digestion and chromatography-free purifications and demonstrates the possibility of cyclohexane-to-benzene conversions under relatively mild, metal-free conditions. Disciplines Materials Chemistry | Other Chemistry | Physical Chemistry Comments Reprinted (adapted) with permission from J. Org. Chem., 2014, 79 (11), pp 5334–5337. Copyright 2014 American Chemical Society. -

Synthesis of 1,2,4 Oxadiazol-5-Imine, 1,2,4-Triazol-3-Imine and Derivatives: a Substituted Cyanamide-Based Strategy for Heterocycle Synthesis
Synthesis of 1,2,4 oxadiazol-5-imine, 1,2,4-triazol-3-imine and derivatives: A Substituted Cyanamide-based Strategy for Heterocycle Synthesis Shreesha V. Bhat A thesis submitted in partial fulfilment of the requirements of the University of Lincoln for the degree of Doctor of Philosophy June 2017 Statement of Originality “I, Shreesha V. Bhat, hereby declare that this submission is my own work and to the best of my knowledge it contains no materials previously published or written by another person, or substantial proportions of material which have been published or accepted for the award of any other degree or diploma at University of Lincoln or any other educational institution, except where references have been made in the thesis. Any contribution made to the research by others, with whom I have worked at the University of Lincoln or elsewhere, is explicitly acknowledged in the thesis. I also declare that the intellectual content of this thesis is the product of my own work, except to the extent that assistance from others in the project's design and conception or in style, presentation and linguistic expression is acknowledged.” (Shreesha V. Bhat) ii | P a g e Abstract Considering the importance of nitrogen-rich heterocycles in drug discovery, a novel strategy towards heterocycle synthesis was envisioned using cyanamide chemistry. Synthesis which involve mild conditions, avoids multi-step sequence and non-toxic reagents are desirable for generation of large combinatorial libraries of drug molecules. We envisaged that the NCN linkage of the cyanamide as well as the concomitant use of the nucleo-and electrophilic centres of the cyanamide could provide a novel synthetic route towards nitrogen heterocycles. -

(12) United States Patent (10) Patent No.: US 8.475,747 B1 Johnson Et Al
USOO8475747B1 (12) United States Patent (10) Patent No.: US 8.475,747 B1 Johnson et al. (45) Date of Patent: Jul. 2, 2013 (54) PROCESSING FISSILE MATERAL (56) References Cited MIXTURES CONTAINING ZIRCONUM AND/OR CARBON U.S. PATENT DOCUMENTS 3,012,849 A 12, 1961 Horn ................................. 423.4 Inventors: Michael Ernest Johnson, Richland, WA 3,965,237 A * 6/1976 Paige .......... ... 423f4 (75) 2003. O156675 A1* 8, 2003 Venneri et al. ... 376/189 (US); Martin David Maloney, 2003/0234223 A1* 12/2003 Kuraoka et al. .... ... 210,660 Evergreen, CO (US) 2007,0290.178 A1* 12/2007 Baron et al. ....... ... 252/643 2008/022410.6 A1* 9, 2008 Johnson et al. ... 252/625 (73) Assignee: U.S. Department of Energy, 2010/0314592 A1* 12/2010 Bourg et al. .................. 252,636 Washington, DC (US) OTHER PUBLICATIONS General Atomics & US DOE, “Development Plan for Advanced High (*) Notice: Subject to any disclaimer, the term of this Temperature Coated-Particle Fuels'. http://nuclearinl.gov/ patent is extended or adjusted under 35 deliverables/docs/pc-000513 0 relpdf, 2003.* U.S.C. 154(b) by 784 days. Pereira, Candido. “UREX-- Process Overview” www.ne.doe.gov/ pdfFiles/DOENRCUREXSeminar.pdfSimilar, Mar. 26, 2008.* Del Cul et al. “TRISO Coated Fuel Processing to Support High (21) Appl. No.: 12/484,561 Temperature Gas-Cooled Reactors.” http://nuclear.gov/peis/refer ences/RM923 DelCuletal 2002.pdf, p. 1-62, Mar. 2002.* (22) Filed: Jun. 15, 2009 Minato et al. “Retention of fission product caesium in ZrC-coated fuel particles for high-temperature gas-cooled reactors.” J. Nuclear Materials, 279, pp. -

Part One Amino Acids As Building Blocks
Part One Amino Acids as Building Blocks Amino Acids, Peptides and Proteins in Organic Chemistry. Vol.3 – Building Blocks, Catalysis and Coupling Chemistry. Edited by Andrew B. Hughes Copyright Ó 2011 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim ISBN: 978-3-527-32102-5 j3 1 Amino Acid Biosynthesis Emily J. Parker and Andrew J. Pratt 1.1 Introduction The ribosomal synthesis of proteins utilizes a family of 20 a-amino acids that are universally coded by the translation machinery; in addition, two further a-amino acids, selenocysteine and pyrrolysine, are now believed to be incorporated into proteins via ribosomal synthesis in some organisms. More than 300 other amino acid residues have been identified in proteins, but most are of restricted distribution and produced via post-translational modification of the ubiquitous protein amino acids [1]. The ribosomally encoded a-amino acids described here ultimately derive from a-keto acids by a process corresponding to reductive amination. The most important biosynthetic distinction relates to whether appropriate carbon skeletons are pre-existing in basic metabolism or whether they have to be synthesized de novo and this division underpins the structure of this chapter. There are a small number of a-keto acids ubiquitously found in core metabolism, notably pyruvate (and a related 3-phosphoglycerate derivative from glycolysis), together with two components of the tricarboxylic acid cycle (TCA), oxaloacetate and a-ketoglutarate (a-KG). These building blocks ultimately provide the carbon skeletons for unbranched a-amino acids of three, four, and five carbons, respectively. a-Amino acids with shorter (glycine) or longer (lysine and pyrrolysine) straight chains are made by alternative pathways depending on the available raw materials. -

Step Polymerization
Chapter 6 Step Polymerization The step polymerization builds up the molecular weight of polymer by stepwise function. Sometimes, the polymerization involves the release of small molecule by-product, so it is also called condensation polymerization. It is the earliest polymerization technique in the synthetic polymers. In 1907, Leo Baekeland of Germany created the first completely synthetic polymer, Bakelite, by reacting phenol and formaldehyde. It is also called phenolic resin. The molecular weight of the phenolic resin builds up stepwise by removing water. The product was com- mercialized in 1909 by forming a company bearing his name as Bakelite until present day. Table 6.1 lists some of the commercially important polymers prepared by step- reaction polymerization. The reaction mechanisms, kinetics of polyesters and polyamides have been thoroughly studied. Thus, we are discussing the degree of polymerization DP and the polymerization rate of step polymerization using these two polymers as examples. 6.1 Chemical Reactions and Reaction Mechanisms of Step Polymerization The type of products formed in step polymerization is determined by the func- tionality of the monomers, i.e., by the average number of reactive functional groups per monomer molecule. Monofunctional monomers give only low molecular weight products. Bifunctional monomers give linear polymers. Poly- functional monomers, with more than two functional groups per molecule, give branched or crosslinked polymers. The properties of the linear and the crosslinked polymers differ widely. -

Combustion of Organic Molecules by the Thermal Decomposition of Perchlorate Salts: Implications for Organics at the Mars Phoenix Scout Landing Site
COMBUSTION OF ORGANIC MOLECULES BY THE THERMAL DECOMPOSITION OF PERCHLORATE SALTS: IMPLICATIONS FOR ORGANICS AT THE MARS PHOENIX SCOUT LANDING SITE. D. W. Ming1, H. V. Lauer, Jr.2, P. D. Archer, Jr.3, B. Sutter4, D. C. Golden5, R. V. Morris1, P. B. Niles1, and W. V. Boynton4; 1NASA Johnson Space Center, Houston, TX 77058 ([email protected]), 2ESCG/Barrios Tech., Houston, TX, 3Lunar and Planetary Laboratory, University of Arizona, Tucson, AZ, 4Jacobs/ESCG, Houston, TX, 5ESCG/Hamilton Sundstrand, Houston, TX. Introduction: The Mars 2007 Phoenix Scout Mis- testbed to understand the effects of the thermal de- sion successfully landed on May 25, 2008 and oper- composition of perchlorate salts on organic molecules. ated on the northern plains of Mars for 150 sols. The Materials and Methods: A three component mix- primary mission objective was to study the history of ture was designed to simulate the thermal decomposi- water and evaluate the potential for past and present tion of perchlorate salt in the presence of organic mate- habitability in Martian arctic ice-rich soil [1]. Phoenix rial. The mixture consisted of 2.3 wt. % Mg- landed near 68° N latitude on polygonal terrain created perchlorate (Mg(ClO4)2.6H2O, 8.4 wt. % mellitic acid, by ice layers that are a few centimeters under loose and 87.3 wt. % SiO2. Mg-perchlorate was chosen be- soil materials. The Phoenix Mission is assessing the cause it is a leading candidate for the perchlorate salt potential for habitability by searching for organic in the Phoenix soils [3]. Mellitic acid (benzenehex- molecules in the ice or icy soils at the landing site. -

Deep UV Raman Spectroscopy for Planetary Exploration: the Search for in Situ Organics
Icarus 290 (2017) 201–214 Contents lists available at ScienceDirect Icarus journal homepage: www.elsevier.com/locate/icarus Deep UV Raman spectroscopy for planetary exploration: The search for in situ organics ∗ William J. Abbey a, Rohit Bhartia a, , Luther W. Beegle a, Lauren DeFlores a, Veronica Paez b, Kripa Sijapati c, Shakher Sijapati d, Kenneth Williford a, Michael Tuite a, William Hug c, Ray Reid c a Jet Propulsion Laboratory, California Institute of Technology, 4800 Oak Grove Drive Ave, Pasadena, CA 91109, United States b Georgia Institute of Technology, North Ave NW, Atlanta, GA, 30332, United States c Photon Systems Inc.,1512 W Industrial Park St, Covina, CA 91722, United States d University of Wisconsin-Madison, Madison, WI 53706, United States a r t i c l e i n f o a b s t r a c t Article history: Raman spectroscopy has emerged as a powerful, non-contact, non-destructive technique for detection and Received 26 September 2016 characterization of in situ organic compounds. Excitation using deep UV wavelengths ( < 250 nm), in par- Revised 24 January 2017 ticular, offers the benefits of spectra obtained in a largely fluorescence-free region while taking advantage Accepted 26 January 2017 of signal enhancing resonance Raman effects for key classes of organic compounds, such as the aromatics. Available online 1 March 2017 In order to demonstrate the utility of this technique for planetary exploration and astrobiological appli- Keywords: cations, we interrogated three sets of samples using a custom built Raman instrument equipped with a Astrobiology deep UV (248.6 nm) excitation source. -

Options for Treatment of Legacy and Advanced
OPTIONS FOR TREATMENT OF LEGACY AND ADVANCED NUCLEAR FUELS A thesis submitted to the University of Manchester for the degree of in the Faculty of Engineering and Physical Sciences. 2014 CHRIS MAHER SCHOOL OF CHEMICAL ENGINEERING AND ANALYTICAL SCIENCE TABLE OF CONTENTS 1. INTRODUCTION ............................................................................ 22 2. OVERALL OBJECTIVES .................................................................. 26 3. TECHNOLOGY FOR THE HEAD-END TREATMENT OF LEGACY, CURRENT AND FUEL ADVANCED NUCLEAR FUELS ......................... 27 3.1. Aims and objectives .................................................................. 27 3.2. Science and technology of Head-End and influence of potential future challenges ............................................................................... 27 3.2.1. Introduction ..................................................................... 28 3.2.2. Current practice ................................................................ 31 3.2.2.1. Fuel conditioning ....................................................... 32 3.2.2.2. Dissolution ............................................................... 33 3.2.2.3. Dissolver liquor conditioning ....................................... 40 3.2.2.4. Accountancy and buffer storage .................................. 42 3.2.3. Potential developments to meet future challenges ................. 43 3.2.3.1. Dissolution chemistry of alternative fuel types .............. 44 3.2.3.2. Fuel conditioning - potential benefits -

Mild Reductive Functionalization of Amides Into N‐Sulfonylformamidines
http://www.diva-portal.org This is the published version of a paper published in ChemistryOpen. Citation for the original published paper (version of record): Trillo, P., Slagbrand, T., Tinnis, F., Adolfsson, H. (2017) Mild Reductive Functionalization of Amides into N-Sulfonylformamidines. ChemistryOpen, 6(4): 484-487 https://doi.org/10.1002/open.201700087 Access to the published version may require subscription. N.B. When citing this work, cite the original published paper. Permanent link to this version: http://urn.kb.se/resolve?urn=urn:nbn:se:umu:diva-138585 DOI:10.1002/open.201700087 Mild Reductive Functionalization of Amides into N- Sulfonylformamidines Paz Trillo,[a] Tove Slagbrand,[a] Fredrik Tinnis,*[a] and Hans Adolfsson*[a, b] The development of aprotocolfor the reductivefunctionaliza- tion of amides into N-sulfonylformamidines is reported. The one-pot procedure is based on amild catalytic reduction of tertiaryamides into the corresponding enamines by the use of Mo(CO)6 (molybdenum hexacarbonyl) and TMDS (1,1,3,3-tetra- methyldisiloxane). The formed enamines were allowed to react with sulfonyl azidestogive the target compounds in moderate to good yields. The amidine functional group is frequently found in biological- ly activecompounds possessing anti-inflammatory,antibacteri- al, antiviral, antibiotic, and anestheticproperties.[1] They are also employed as intermediates and precursors in organic syn- thesis of importantheterocyclic compounds such as imida- zoles, quinazolines,isoquinolines, and pyrimidines.[2] Further- more, amidines are employed as ligandsinmetal complexes and as protecting groups for primary amines.[3] Scheme1.Preparation of amidines through a–c) electrophilic amide activa- The stability of amides makes this functional group valuable tion and d) reductive functionalization of amides. -

The Missing Organic Molecules on Mars
The missing organic molecules on Mars Steven A. Benner*, Kevin G. Devine, Lidia N. Matveeva, and David H. Powell Departments of Chemistry, Anatomy, and Cell Biology, University of Florida, Gainesville, FL 32611 Communicated by Leslie Orgel, The Salk Institute for Biological Studies, San Diego, CA, December 13, 1999 (received for review November 4, 1998) GC-MS on the Viking 1976 Mars missions did not detect organic and with the irradiation of the surface by ultraviolet light, the molecules on the Martian surface, even those expected from failure to detect organic substances led many to conclude that meteorite bombardment. This result suggested that the Martian one must dig deeply (and perhaps very deeply) below the regolith might hold a potent oxidant that converts all organic Martian surface to have a chance of encountering any organic molecules to carbon dioxide rapidly relative to the rate at which molecules that may have arisen from life on Mars (perhaps they arrive. This conclusion is influencing the design of Mars present several billion years ago, when the surface of Mars was missions. We reexamine this conclusion in light of what is known more like the surface of Earth at that time and when life almost about the oxidation of organic compounds generally and the certainly had emerged on Earth) or of encountering organic nature of organics likely to come to Mars via meteorite. We molecules that may have been delivered to Mars via meteorite conclude that nonvolatile salts of benzenecarboxylic acids, and (9, 10). perhaps oxalic and acetic acid, should be metastable intermediates Because this interpretation is influencing the design of mis- of meteoritic organics under oxidizing conditions. -

Degradable Chelants Having Sulfonate Groups, Uses and Compositions Thereof
Europa,schesP_ MM II M M MM I MM MM M M I M J European Patent Office «• o - B4 © Publication number: 0 516 102 B1 Office_„. europeen des brevets © EUROPEAN PATENT SPECIFICATION © Date of publication of patent specification: 10.05.95 © Int. CI.6: C07C 309/14, C1 1 D 3/34 © Application number: 92108980.1 @ Date of filing: 27.05.92 Degradable chelants having sulfonate groups, uses and compositions thereof. © Priority: 31.05.91 US 708534 © Proprietor: THE DOW CHEMICAL COMPANY 2030 Dow Center, @ Date of publication of application: Abbott Road 02.12.92 Bulletin 92/49 Midland, Michigan 48640 (US) © Publication of the grant of the patent: 10.05.95 Bulletin 95/19 @ Inventor: Crump, Druce K. 142 Oyster Creek Drive, Number 48 © Designated Contracting States: Lake Jackson, AT BE CH DE DK ES FR GB GR IT LI LU MC Texas 77566 (US) NL PT SE Inventor: Wilson, David A. 229 San Saba © References cited: Richwood, DE-A- 3 337 026 Texas 77531 (US) US-A- 3 091 522 US-A- 3 703 545 US-A- 3 726 912 © Representative: Huber, Bernhard, Dlpl.-Chem. et al Patentanwalte H. Weickmann, Dr. K. Fincke F.A. Weickmann, B. Huber Dr. H. Liska, Dr. J. Prechtel, Dr. B. Bohm Postfach 86 08 20 CD D-81635 Munchen (DE) CM O CO lo Note: Within nine months from the publication of the mention of the grant of the European patent, any person ® may give notice to the European Patent Office of opposition to the European patent granted. Notice of opposition Q,. -

United States Patent Office Patented Jan
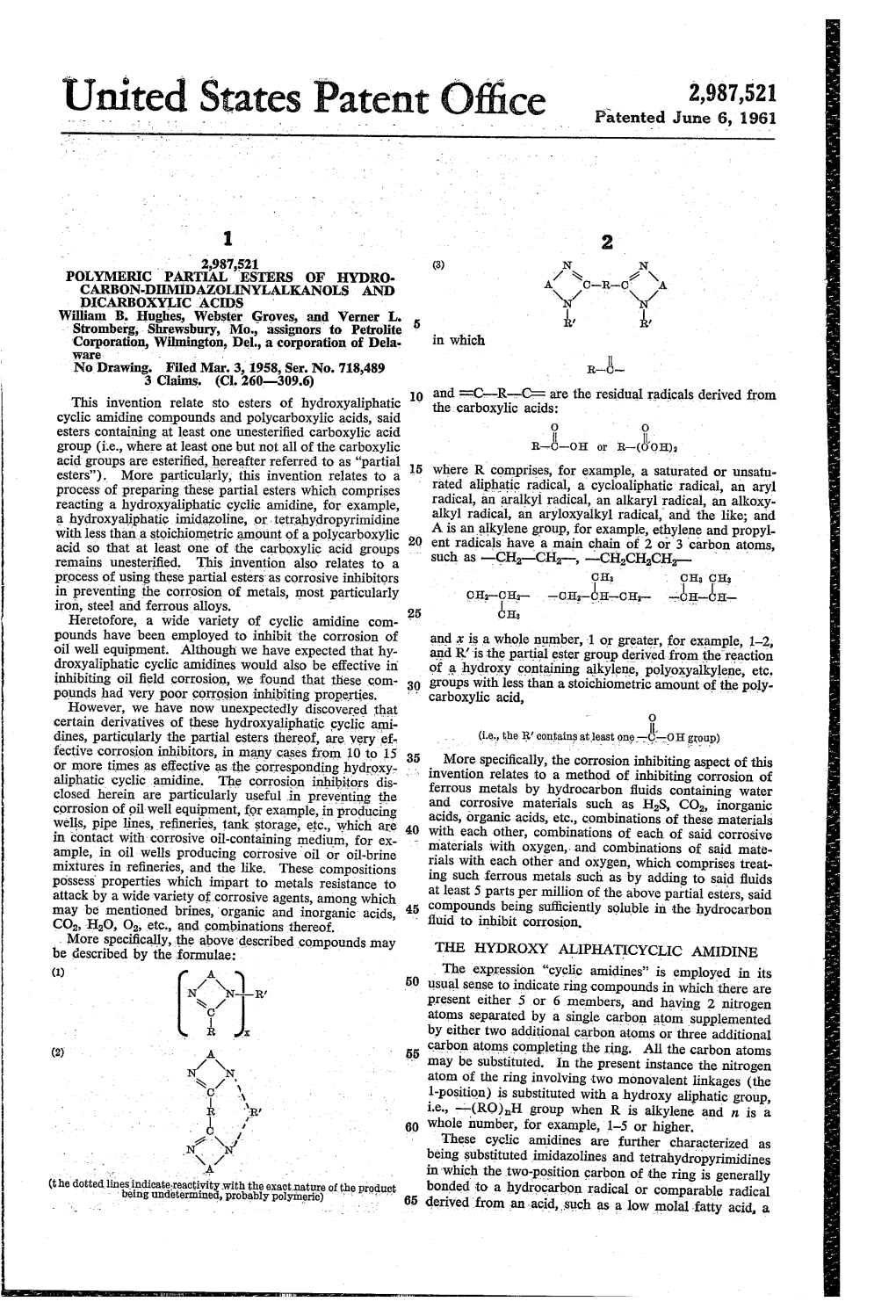
3,017,356 United States Patent Office Patented Jan. 16, 1962 1. 2 rated aliphatic radical, a cycloaliphatic radical, an aryl 3,017,356 radical, an aralkyl radical, an alkaryl radical, an alkoxy WillianPROCESS B. Hughes, OF andINHIBITING Verner L. Stronberg,CORROSION Webster alkyl radical, an aryloxyalkyl radical, and the like; and Groves, Mo., assignor's to Petroite Corporation, Wii A is an alkylene group; for example, ethylene and propyl mington, Del, a corporation of Delaware ene radicals, such as No Drawing. Original application Mar. 3, 1958, Ser. No. 718,391 Divided and this application Nov. 23, 1959, Ser. No. 854,553 -CH2CH2CH 15 Claims. (C. 252-8.55) CH-CH2 O B This application is a division of Serial No. 718,391, CHs filed March 3, 1958. This invention relates to esters of cyclic amidines of -CH-CH-CH,- the formula gh, CHis O 15 -bH-bH In general, the amidine esters are prepared by reacts o-o-c- ing a hydroxyaliphatic cyclic amidine ()-ROH with less where (A) and (B) are cyclic amidine-containing radicals, than a stoichiometric amount of a polycarboxylic acid to for example, imidazoline and tetrahydropyrimidine radi form a half ester cals (hereafter referred to as “amidine esters'). More 20 particularly, this invention relates to esters wherein. A contains one type of cyclic amidine ring and B contains (a)-R-O C-Z-COH the same or another type not selected in A. This inven which is subsequently reacted with an amidine forming tion also relates to a process of preparing these com polyamine to form the amidine ester pounds which comprises reacting a hydroxy-containing 25 O cyclic amidine with less than a stoichiometric amount of a polycarboxylic acid to form a partial ester and then re Q-R-O-)-2- acting this partial ester with a polyamine capable of form More specifically, the corrosion inhibiting aspect of this ing a second amidine ring of the same or different type.