Brief Report
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

22-556Orig1s000
CENTER FOR DRUG EVALUATION AND RESEARCH APPLICATION NUMBER: 22-556Orig1s000 MEDICAL REVIEW(S) MEDICAL OFFICER REVIEW Division Of Pulmonary, Allergy, and Rheumatology Products, HFD-570 APPLICATION: NDA 22-556 TRADE NAME: Karbinal ER™ APPLICANT/SPONSOR: Tris Pharma USAN NAME: Carbinoxamine Extended-Release MEDICAL OFFICER: Peter Starke, MD Oral Suspension TEAM LEADER: Theresa Michele, MD CATEGORY: Antihistamine DATE: February 25, 2013 ROUTE: Oral SUBMISSIONS REVIEWED IN THIS DOCUMENT Document Date Submission Date Application/Doc Comments October 4, 2012 October 5, 2012 SD-17 Complete Response submission January 8, 2013 January 9, 2013 SD-20 Response to labeling (formatting) IR RELATED APPLICATIONS Date Application Comments REVIEW SUMMARY: This is clinical review of a Complete Response (CR) to a CR action taken by the Agency on October 7, 2011, for a 505(b)(2) application from Tris Pharma for Carbinoxamine Extended-Release (ER) Oral Suspension, equivalent to 4 mg of carbinoxamine maleate (CM) per 5 mL. The formulation is a sustained release formulation of carbinoxamine maleate suspended in a drug-polistirex resin complex. The proposed Trade Name is Karbinal ER. The application references both the currently available generic immediate-release Carbinoxamine Maleate 4 mg tablets (ANDA 40-442) and oral solution 4 mg/5 mL(ANDA 40-458), marketed under the brand name Palgic and manufactured by Milkart, Inc., and the no-longer-marketed immediate-release innovator products, Clistin 4 mg tablets (NDA 08-915) and 4 mg/5 mL elixir (NDA 08-955), previously marketed by McNeil. McNeil discontinued marketing the Clistin products in the 1990s, and the Orange Book makes the notation that the Clistin products were not discontinued or withdrawn for safety or efficacy reasons. -

XYZAL (Levocetirizine Dihydrochloride)
HIGHLIGHTS OF PRESCRIBING INFORMATION • Patients with end-stage renal impairment at less than 10 mL/min These highlights do not include all the information needed to use XYZAL creatinine clearance or patients undergoing hemodialysis (2.3, 4) safely and effectively. See full prescribing information for XYZAL. • Children 6 to 11 years of age with renal impairment (2.3, 4) ® XYZAL (levocetirizine dihydrochloride) ------------------------WARNINGS AND PRECAUTIONS----------------------- 5 mg tablets 2.5 mg/5 mL (0.5 mg/mL) oral solution • Avoid engaging in hazardous occupations requiring complete mental Initial U.S. Approval: 1995 alertness such as driving or operating machinery when taking XYZAL (5.1). ---------------------------RECENT MAJOR CHANGES--------------------------- • Avoid concurrent use of alcohol or other central nervous system Dosage and Administration (2) 01/2008 depressants with XYZAL (5.1). Dosage and Administration, Adults & Children 12 Years & Older (2.1) 01/2008 ------------------------------ADVERSE REACTIONS------------------------------ Dosage and Administration, Children 6 to 11 Years (2.2) 01/2008 The most common adverse reactions (rate ≥2% and > placebo) were ---------------------------INDICATIONS AND USAGE---------------------------- somnolence, nasopharyngitis, fatigue, dry mouth, and pharyngitis in subjects XYZAL is a histamine H1-receptor antagonist indicated for: 12 years of age and older, and pyrexia, somnolence, cough, and epistaxis in • The relief of symptoms associated with seasonal and perennial allergic -

FEXOFENADINE HYDROCHLORIDE 180 MG FILM-COATED TABLETS Fexofenadine Hydrochloride
ID1089 MRP _ UK Version: 07 Review Date: 19/02/2020 PACKAGE LEAFLET: INFORMATION FOR THE USER FEXOFENADINE HYDROCHLORIDE 120 MG FILM-COATED TABLETS FEXOFENADINE HYDROCHLORIDE 180 MG FILM-COATED TABLETS Fexofenadine hydrochloride Read all of this leaflet carefully before you start using this medicine because it contains important information for you. - Keep this leaflet. You may need to read it again. - If you have any further questions, ask your doctor or pharmacist. - This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours. - If you get any of the side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet, (see section 4). In this leaflet: 1. What Fexofenadine hydrochloride is and what it is used for 2. What you need to know before you take Fexofenadine hydrochloride 3. How to take Fexofenadine hydrochloride 4. Possible side effects of Fexofenadine hydrochloride 5. How to store Fexofenadine hydrochloride 6. Contents of the pack and other information 1. WHAT FEXOFENADINE HYDROCHLORIDE IS AND WHAT IT IS USED FOR FEXOFENADINE HYDROCHLORIDE Contains fexofenadine hydrochloride which is an antihistamine. Only Fexofenadine hydrochloride 120 mg tablets is used in adults and adolescents of 12 years and older to relieve the symptoms that occur with hay fever (seasonal allergic rhinitis) such as sneezing, itchy, running or blocked nose and itchy, red and watery eye). Fexofenadine hydrochloride 180 mg tablets is used in adults and adolescents of 12 years and older to relieve the symptoms that occur with long term allergic skin reactions( chronic idiopathic urticaria) such as itching, swelling and rashes 2. -

New York State Medicaid Drug Utilization Review Board Meeting Agenda
New York State Medicaid Drug Utilization Review Board Meeting Agenda The Drug Utilization Review (DUR) Board will meet on April 27, 2016, from 9:00 a.m. to 4:00 p.m., Meeting Room 6, Concourse, Empire State Plaza, Albany, New York Agenda Items A. Preferred Drug Program (PDP) The DUR Board will review therapeutic classes listed below, as they pertain to the PDP. The DUR Board will review clinical and financial information, to recommend preferred and non-preferred drugs. For therapeutic classes currently subject to the PDP^, the DUR Board will only consider clinical information which is new since the previous review of the therapeutic class and then consider financial information. New clinical information may include a new drug or drug product information, new indications, new safety information or new published clinical trials (comparative evidence is preferred, or placebo controlled when no head-to-head trials are available). Information in abstract form alone, posters, or unpublished data are poor quality evidence for the purpose of re-review and submission is discouraged. Those wishing to submit new clinical information must do so in an electronic format by April 12, 2016 or the Board may not have ample time to review the information. ^ The current preferred and non-preferred status of drugs subject to the Preferred Drug List (PDL) may be viewed at https://newyork.fhsc.com/downloads/providers/NYRx_PDP_PDL.pdf 1. Non-Steroidal Anti-Inflammatory Drugs (NSAIDS) – Prescription (Previous review date: April 22, 2015) Anaprox DS (naproxen -

Potential Drug-Drug Interactions and Adverse Drug Reactions Associated with Hydroxychloroquine
Original Article Potential Drug-drug Interactions and Adverse Drug Reactions Associated with Hydroxychloroquine Arjun Singh1, Richa Chaudhary1, Prayas Verma2, Nilanchal Trivedi3,*, Md. Shamim4 1Department of Pharmacy Practice, Teerthanker Mahaveer College of Pharmacy, TMU, Moradabad, Uttar Pradesh, INDIA. 2Teerthanker Mahaveer Dental College and Research Centre, TMU Moradabad, Uttar Pradesh, INDIA. 3Department of Pharmacology, Teerthanker Mahaveer College of Pharmacy, TMU, Moradabad, Uttar Pradesh, INDIA. 4LCP College of Pharmacy Baghpat, Abdul Kalam Technical University (AKTU), Uttar Pradesh, INDIA. ABSTRACT Introduction: COVID-19 is a pandemic disaster and a health emergency of prime focus for all the world economies. Various prophylactic treatments are considered to combat the disease. Hydroxychloroquine drug is one such option that is given much attention as an armor against SARS COV-2 pandemic. Evaluation and assessment of drug interactions and ADRs is required from ethical concern to justify the use of HCQ on such large scale. Methods: We have performed an analysis of HCQ drug interactions on Micromedex®. We have reviewed literature of HCQ pharmacokinetic properties, ADRs/ ADEs and toxicities associated with the use of HCQ drug on PubMed, Google Scholar and CDC database. Results: There are around 180 drug interactions possible with HCQ. Out of them 13 are of contraindicated severity level and other 165 are of major severity and 2 of them are moderately severe. Most of the interactions are coupled with QT prolonging agents (170), Cardiac arrhythmias is possible with the concomitant use of at least 2 drugs, 4 drugs leads to Torsade de points. System organ level ADRs are also evaluated along with various precautions, warnings and contraindications. -
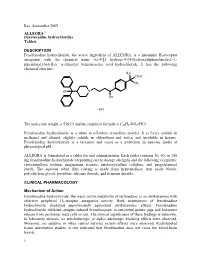
Fexofenadine Hydrochloride) Tablets
Rev. September 2005 ALLEGRA® (fexofenadine hydrochloride) Tablets DESCRIPTION Fexofenadine hydrochloride, the active ingredient of ALLEGRA, is a histamine H1-receptor antagonist with the chemical name (±)-4-[1 hydroxy-4-[4-(hydroxydiphenylmethyl)-1- piperidinyl]-butyl]-α, α-dimethyl benzeneacetic acid hydrochloride. It has the following chemical structure: The molecular weight is 538.13 and the empirical formula is C32H39NO4•HCl. Fexofenadine hydrochloride is a white to off-white crystalline powder. It is freely soluble in methanol and ethanol, slightly soluble in chloroform and water, and insoluble in hexane. Fexofenadine hydrochloride is a racemate and exists as a zwitterion in aqueous media at physiological pH. ALLEGRA is formulated as a tablet for oral administration. Each tablet contains 30, 60, or 180 mg fexofenadine hydrochloride (depending on the dosage strength) and the following excipients: croscarmellose sodium, magnesium stearate, microcrystalline cellulose, and pregelatinized starch. The aqueous tablet film coating is made from hypromellose, iron oxide blends, polyethylene glycol, povidone, silicone dioxide, and titanium dioxide. CLINICAL PHARMACOLOGY Mechanism of Action Fexofenadine hydrochloride, the major active metabolite of terfenadine, is an antihistamine with selective peripheral H1-receptor antagonist activity. Both enantiomers of fexofenadine hydrochloride displayed approximately equipotent antihistaminic effects. Fexofenadine hydrochloride inhibited antigen-induced bronchospasm in sensitized guinea pigs and histamine -

MEDICATIONS to AVOID PRIOR to ALLERGY SKIN TESTING Allergy
MEDICATIONS TO AVOID PRIOR TO ALLERGY SKIN TESTING Allergy testing requires the ‘histamine response’ in order to be accurate and reliable. There are many types of antihistamines. Antihistamines are found in many different medicines, either as a single drug or mixed with a combination of chemicals. Please review all medicines you take (including Over-The-Counter) in order to make your allergy testing appointment most efficient and accurate. Generic names are in all lower case, trade names Capitalized. Oral antihistamines to be stopped 3 (THREE) days prior to your appointment: - brompheniramine (Actifed, Atrohist, Dimetapp, Drixoral) - cetirizine (Zyrtec, Zyrtec D) - chlopheniramine (Chlortrimeton, Deconamine, Kronofed A, Novafed A, Rynatan, Tussinex) - clemastine (Tavist, Antihist) - cyproheptadine (Periactin) - diphenhydramine (Benadryl, Allernix, Nytol) - doxylamine (Bendectin, Nyquil) - hydroxyzine (Atarax, Marax, Vistaril) - levocetirizine (Xyzal) - promethazine (Phenergan) Oral antihistamines to be stopped 7 (SEVEN) days prior to your appointment: - desloratadine (Clarinex) - fexofenadine (Allegra, Allegra D) - loratadine (Claritin, Claritin D, Alavert) Nose spray and eye drop antihistamines to stop 5 (FIVE) days prior to your appointment: - azelastine (Astelin, Astepro, Dymista, Optivar) - bepotastine (Bepreve) - ketotifen (Zaditor, Alaway) - olapatadine (Pataday, Patanase) - pheniramine (Visine A, Naphcon A) – OK to stop for 2 days Antacid medications (different type of antihistamine) to stop 3 (THREE) days prior to your appointment: - cimetidine (Tagamet) - famotidine (Pepcid) - ranitidine (Zantac) Note: Antihistamines are found in many over the counter medications, including Tylenol Allergy, Actifed Cold and Allergy, Alka-Seltzer Plus Cold with Cough Formula, and many others. Make sure you read and check the ingredients carefully and stop those containing antihistamines at least 3 (THREE) days prior to the appointment. -

Prescribing Information for High-Dose Fexofenadine in the Management of Urticaria in Adults
1 Prescribing information for high-dose fexofenadine in the management of urticaria in adults This prescribing information document outlines the prescribing responsibilities between the specialist and GP. GPs are invited to participate. If the GP feels that such prescribing is outside their area of expertise or have clinical concerns about the safe management of the drug in primary care, then he or she is under no obligation to do so. In such an event, clinical responsibility for the patient’s health remains with the specialist. If a specialist asks the GP to prescribe, the GP should reply to this request as soon as practicable. Consultant details GP details Patient details Name: Name: Name: Address: Address: NHS Number: Email: Email: Date of birth: Contact number: Contact number: Contact: Introduction Fexofenadine is a second generation non-sedating H1-antihistamine used in the treatment of allergic disorders. Fexofenadine is a pharmacologically active metabolite of terfenadine. Licensed indication: In adults and children 12 years and older, the licensed dose is 180mg daily for the relief of symptoms associated with chronic idiopathic urticaria. Unlicensed indication (the focus of this document): As advised by Europeani and British Association of Dermatologistsii guidelines, doses of antihistamines up to four times the recommended daily dose may be used in the treatment of urticaria. Adult dosage and administration The licensed maximum recommended daily dose is 180mg once daily taken before a meal. Doses of up to four times the daily recommended dose have been used in the treatment of severe urticaria (unlicensed indication)i Available as: 30mg, 120mg and 180mg tablets Specialist responsibilities Provide patient/carer with relevant written information on the unlicensed use of high-dose fexofenadine and possible side-effects. -

Guideline for Preoperative Medication Management
Guideline: Preoperative Medication Management Guideline for Preoperative Medication Management Purpose of Guideline: To provide guidance to physicians, advanced practice providers (APPs), pharmacists, and nurses regarding medication management in the preoperative setting. Background: Appropriate perioperative medication management is essential to ensure positive surgical outcomes and prevent medication misadventures.1 Results from a prospective analysis of 1,025 patients admitted to a general surgical unit concluded that patients on at least one medication for a chronic disease are 2.7 times more likely to experience surgical complications compared with those not taking any medications. As the aging population requires more medication use and the availability of various nonprescription medications continues to increase, so does the risk of polypharmacy and the need for perioperative medication guidance.2 There are no well-designed trials to support evidence-based recommendations for perioperative medication management; however, general principles and best practice approaches are available. General considerations for perioperative medication management include a thorough medication history, understanding of the medication pharmacokinetics and potential for withdrawal symptoms, understanding the risks associated with the surgical procedure and the risks of medication discontinuation based on the intended indication. Clinical judgement must be exercised, especially if medication pharmacokinetics are not predictable or there are significant risks associated with inappropriate medication withdrawal (eg, tolerance) or continuation (eg, postsurgical infection).2 Clinical Assessment: Prior to instructing the patient on preoperative medication management, completion of a thorough medication history is recommended – including all information on prescription medications, over-the-counter medications, “as needed” medications, vitamins, supplements, and herbal medications. Allergies should also be verified and documented. -

Urticaria: Current Opinions About Etiology, Diagnosis and Therapy
Acta Derm Venereol 2007; 87: 196–205 REVIEW ARTICLE Urticaria: Current Opinions about Etiology, Diagnosis and Therapy Torsten ZUBERBIER and Marcus MAURER Department of Dermatology and Allergy, Allergie-Centrum-Charité, Charité – Universitätsmedizin, Berlin, Germany In the last few decades an increasing understanding of British Association of Dermatologists (4) and the most the pathomechanisms involved in urticaria has highlight recent European Academy of Allergology and Clinical ed the heterogeneity of different subtypes. According to Immunology/Global Allergy and Asthma European Net- the new European Academy of Allergology and Clinical work/European Dermatology Forum (EAACI/GA²LEN/ Immunology/Global Allergy and Asthma European Net EDF) guidelines. For preparing the latter, more than work/European Dermatology Forum (EAACI/GA²LEN/ 300 specialists from over 15 countries held a consensus EDF) guidelines, urticaria subtypes can be grouped into meeting regarding the definition, classification, routine spontaneous urticaria, which includes acute urticaria diagnosis and management of urticaria. Evidence-ba- and chronic urticaria, the physical urticarias, and other sed suggestions, prepared by a panel in advance, were urticaria disorders, including, for example, contact ur discussed using a voting system (5, 6). ticaria. Clarity of nomenclature is required not only to Due to the heterogeneity of the disease, the interpre- choose the correct measures in diagnosis and manage tation of divergent data from different centres regarding ment, but also to compare data from different studies. eliciting causes in subtypes of urticaria and their therapeu- Urticaria has a profound impact on quality of life and tic responsiveness is, however, sometimes difficult, as the performance. Effective treatment is thus required in all influence of different study populations is considerable. -

Antihistamines in the Treatment of Chronic Urticaria I Jáuregui,1 M Ferrer,2 J Montoro,3 I Dávila,4 J Bartra,5 a Del Cuvillo,6 J Mullol,7 J Sastre,8 a Valero5
Antihistamines in the treatment of chronic urticaria I Jáuregui,1 M Ferrer,2 J Montoro,3 I Dávila,4 J Bartra,5 A del Cuvillo,6 J Mullol,7 J Sastre,8 A Valero5 1 Service of Allergy, Hospital de Basurto, Bilbao, Spain 2 Department of Allergology, Clínica Universitaria de Navarra, Pamplona, Spain 3 Allergy Unit, Hospital La Plana, Villarreal (Castellón), Spain 4 Service of Immunoallergy, Hospital Clínico, Salamanca, Spain 5 Allergy Unit, Service of Pneumology and Respiratory Allergy, Hospital Clínic (ICT), Barcelona, Spain 6 Clínica Dr. Lobatón, Cádiz, Spain 7 Rhinology Unit, ENT Service (ICEMEQ), Hospital Clínic, Barcelona, Spain 8 Service of Allergy, Fundación Jiménez Díaz, Madrid, Spain ■ Summary Chronic urticaria is highly prevalent in the general population, and while there are multiple treatments for the disorder, the results obtained are not completely satisfactory. The second-generation H1 antihistamines remain the symptomatic treatment option of choice. Depending on the different pharmacokinetics and H1 receptor affi nity of each drug substance, different concentrations in skin can be expected, together with different effi cacy in relation to the histamine-induced wheal inhibition test - though this does not necessarily have repercussions upon clinical response. The antiinfl ammatory properties of the H1 antihistamines could be of relevance in chronic urticaria, though it is not clear to what degree they infl uence the fi nal therapeutic result. Before moving on to another therapeutic level, the advisability of antihistamine dose escalation should be considered, involving increments even above those approved in the Summary of Product Characteristics. Physical urticaria, when manifesting isolatedly, tends to respond well to H1 antihistamines, with the exception of genuine solar urticaria and delayed pressure urticaria. -

Original Article Clinical Efficacy of Levocetirizine Combined with Ebastine in the Treatment of Chronic Urticaria and Their Effect on Serum Cytokines
Int J Clin Exp Med 2019;12(9):11675-11683 www.ijcem.com /ISSN:1940-5901/IJCEM0097567 Original Article Clinical efficacy of levocetirizine combined with ebastine in the treatment of chronic urticaria and their effect on serum cytokines Sheng Wang1, Yang Liu2, Jing Wang3, Jing Zhang1 1Department of Dermatology, Tangshan Gongren Hospital, Tangshan 063000, Hebei Province, China; 2Depart- ment of Anesthesiology, Kailuan General Hospital, Tangshan, Hebei Province, China; 3Department of Dermatology, North China University of Science and Technology Affiliated Hospital, Tangshan, Hebei Province, China Received May 27, 2019; Accepted August 6, 2019; Epub September 15, 2019; Published September 30, 2019 Abstract: Objective: To investigate the clinical efficacy of levocetirizine combined with ebastine in the treatment of chronic urticaria and their effect on serum cytokines. Methods: In total, 234 patients with chronic urticaria were selected as the study subjects and divided into the control group and the experimental group, with 117 patients in each group. The experimental group was treated with levocetirizine combined with ebastine, and the control group was treated with levocetirizine. The clinical efficacy and the incidence of adverse reactions were observed after treatment. The levels of serum tumor necrosis factor (TNF)-α, interleukin (IL)-6 and IL-10 were detected by enzyme-linked immunosorbent assay (ELISA). Patients were divided into group A (126 patients, cure) and group B (108 patients, markedly effective, effective and ineffective) according to clinical efficacy, and the serum levels of TNF-α, IL-6 and IL-10 were compared between the two groups. The receiver operating characteristic (ROC) curve was used to analyze the value of TNF-α, IL-6 and IL-10 in evaluating the clinical efficacy after treatment for patients with chronic urticaria.