Zootaxa 3986
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Acte Argeo Final
GEOTHERMAL RESOURCE INDICATIONS OF THE GEOLOGIC DEVELOPMENT AND HYDROTHERMAL ACTIVITIES OF D.R.C. Getahun Demissie Addis Abeba, Ethiopia, [email protected] ABSTRACT Published sources report the occurrence of more than 135 thermal springs in D.R.C. All occur in the eastern part of the country, in association with the Western rift and the associated rifted and faulted terrains lying to its west. Limited information was available on the characteristics of the thermal features and the natural conditions under which they occur. Literature study of the regional distribution of these features and of the few relatively better known thermal spring areas, coupled with the evaluation of the gross geologic conditions yielded encouraging results. The occurrence of the anomalously large number of thermal springs is attributed to the prevalence of abnormally high temperature conditions in the upper crust induced by a particularly high standing region of anomalously hot asthenosphere. Among the 29 thermal springs the locations of which could be determined, eight higher temperature features which occur in six geologic environments were found to warrant further investigation. The thermal springs occur in all geologic terrains. Thermal fluid ascent from depth is generally influenced by faulting while its emergence at the surface is controlled by the near-surface hydrology. These factors allow the adoption of simple hydrothermal fluid circulation models which can guide exploration. Field observations and thermal water sampling for chemical analyses are recommended for acquiring the data which will allow the selection of the most promising prospects for detailed, integrated multidisciplinary exploration. An order of priorities is suggested based on economic and technical criteria. -

Evidence of Hidden Diversity and Taxonomic Conflicts in Five Stream Fishes from the Eastern Zimbabwe Highlands Freshwater Ecoregion
A peer-reviewed open-access journal ZooKeys 768: 69–95Evidence (2018) of hidden diversity and taxonomic conflicts in five stream fishes... 69 doi: 10.3897/zookeys.768.21944 RESEARCH ARTICLE http://zookeys.pensoft.net Launched to accelerate biodiversity research Evidence of hidden diversity and taxonomic conflicts in five stream fishes from the Eastern Zimbabwe Highlands freshwater ecoregion Albert Chakona1,2, Wilbert T. Kadye2, Taurai Bere3, Daniel N. Mazungula1,2, Emmanuel Vreven4,5 1 South African Institute for Aquatic Biodiversity, Private Bag 1015, Grahamstown, South Africa, 6140 2 Department of Ichthyology and Fisheries Science, Rhodes University, P.O. Box 94, Grahamstown, South Africa, 6140 3 School of Wildlife, Ecology and Conservation, Chinhoyi University of Technology, P. Bag 7724, Chinhoyi, Zimbabwe 4 Royal Museum for Central Africa, Section of Vertebrates, Ichthyology, Leuvensesteenweg 13, 3080, Tervuren, Belgium 5 KU Leuven, Department of Biology, Laboratory of Biodiversity and Evolutio- nary Genomics, Deberiotstraat 32, 3000 Leuven, Belgium Corresponding author: Albert Chakona ([email protected]) Academic editor: N. Bogutskaya | Received 30 October 2018 | Accepted 25 April 2018 | Published 19 June 2018 http://zoobank.org/9621930C-8C43-40D0-8554-684035E99FAA Citation: Chakona A, Kadye WT, Bere T, Mazungula DN, Vreven E (2018) Evidence of hidden diversity and taxonomic conflicts in five stream fishes from the Eastern Zimbabwe Highlands freshwater ecoregion. ZooKeys 768: 69–95. https://doi.org/10.3897/zookeys.768.21944 Abstract -
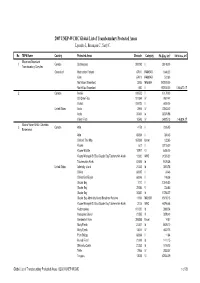
2007 UNEP-WCMC Global List of Transboundary Protected Areas Lysenko I., Besançon C., Savy C
2007 UNEP-WCMC Global List of Transboundary Protected Areas Lysenko I., Besançon C., Savy C. No TBPA Name Country Protected Areas Sitecode Category PA Size, km 2 TBPA Area, km 2 Ellesmere/Greenland 1 Canada Quttinirpaaq 300093 II 38148.00 Transboundary Complex Greenland Hochstetter Forland 67910 RAMSAR 1848.20 Kilen 67911 RAMSAR 512.80 North-East Greenland 2065 MAB-BR 972000.00 North-East Greenland 650 II 972000.00 1,008,470.17 2 Canada Ivvavik 100672 II 10170.00 Old Crow Flats 101594 IV 7697.47 Vuntut 100673 II 4400.00 United States Arctic 2904 IV 72843.42 Arctic 35361 Ia 32374.98 Yukon Flats 10543 IV 34925.13 146,824.27 Alaska-Yukon-British Columbia 3 Canada Atlin 4178 II 2326.95 Borderlands Atlin 65094 II 384.45 Chilkoot Trail Nhp 167269 Unset 122.65 Kluane 612 II 22015.00 Kluane Wildlife 18707 VI 6450.00 Kluane/Wrangell-St Elias/Glacier Bay/Tatshenshini-Alsek 12200 WHC 31595.00 Tatshenshini-Alsek 67406 Ib 9470.26 United States Admiralty Island 21243 Ib 3803.76 Chilkat 68395 II 24.46 Chilkat Bald Eagle 68396 II 198.38 Glacier Bay 1010 II 13045.50 Glacier Bay 22485 V 233.85 Glacier Bay 35382 Ib 10784.27 Glacier Bay-Admiralty Island Biosphere Reserve 11591 MAB-BR 15150.15 Kluane/Wrangell-St Elias/Glacier Bay/Tatshenshini-Alsek 2018 WHC 66796.48 Kootznoowoo 101220 Ib 3868.24 Malaspina Glacier 21555 III 3878.40 Mendenhall River 306286 Unset 14.57 Misty Fiords 21247 Ib 8675.10 Misty Fjords 13041 IV 4622.75 Point Bridge 68394 II 11.64 Russell Fiord 21249 Ib 1411.15 Stikine-LeConte 21252 Ib 1816.75 Tetlin 2956 IV 2833.07 Tongass 13038 VI 67404.09 Global List of Transboundary Protected Areas ©2007 UNEP-WCMC 1 of 78 No TBPA Name Country Protected Areas Sitecode Category PA Size, km 2 TBPA Area, km 2 Tracy Arm-Fords Terror 21254 Ib 2643.43 Wrangell-St Elias 1005 II 33820.14 Wrangell-St Elias 35387 Ib 36740.24 Wrangell-St. -

IMPACTS of CLIMATE CHANGE on WATER AVAILABILITY in ZAMBIA: IMPLICATIONS for IRRIGATION DEVELOPMENT By
Feed the Future Innovation Lab for Food Security Policy Research Paper 146 August 2019 IMPACTS OF CLIMATE CHANGE ON WATER AVAILABILITY IN ZAMBIA: IMPLICATIONS FOR IRRIGATION DEVELOPMENT By Byman H. Hamududu and Hambulo Ngoma Food Security Policy Research Papers This Research Paper series is designed to timely disseminate research and policy analytical outputs generated by the USAID funded Feed the Future Innovation Lab for Food Security Policy (FSP) and its Associate Awards. The FSP project is managed by the Food Security Group (FSG) of the Department of Agricultural, Food, and Resource Economics (AFRE) at Michigan State University (MSU), and implemented in partnership with the International Food Policy Research Institute (IFPRI) and the University of Pretoria (UP). Together, the MSU-IFPRI-UP consortium works with governments, researchers and private sector stakeholders in Feed the Future focus countries in Africa and Asia to increase agricultural productivity, improve dietary diversity and build greater resilience to challenges like climate change that affect livelihoods . The papers are aimed at researchers, policy makers, donor agencies, educators, and international development practitioners. Selected papers will be translated into French, Portuguese, or other languages. Copies of all FSP Research Papers and Policy Briefs are freely downloadable in pdf format from the following Web site: https://www.canr.msu.edu/fsp/publications/ Copies of all FSP papers and briefs are also submitted to the USAID Development Experience Clearing House (DEC) at: http://dec.usaid.gov/ ii AUTHORS: Hamududu is Senior Engineer, Water Balance, Norwegian Water Resources and Energy Directorate, Oslo, Norway and Ngoma is Research Fellow, Climate Change and Natural Resources, Indaba Agricultural Policy Research Institute (IAPRI), Lusaka, Zambia and Post-Doctoral Research Associate, Department of Agricultural, Food and Resource Economics, Michigan State University, East Lansing, MI. -

Fish, Various Invertebrates
Zambezi Basin Wetlands Volume II : Chapters 7 - 11 - Contents i Back to links page CONTENTS VOLUME II Technical Reviews Page CHAPTER 7 : FRESHWATER FISHES .............................. 393 7.1 Introduction .................................................................... 393 7.2 The origin and zoogeography of Zambezian fishes ....... 393 7.3 Ichthyological regions of the Zambezi .......................... 404 7.4 Threats to biodiversity ................................................... 416 7.5 Wetlands of special interest .......................................... 432 7.6 Conservation and future directions ............................... 440 7.7 References ..................................................................... 443 TABLE 7.2: The fishes of the Zambezi River system .............. 449 APPENDIX 7.1 : Zambezi Delta Survey .................................. 461 CHAPTER 8 : FRESHWATER MOLLUSCS ................... 487 8.1 Introduction ................................................................. 487 8.2 Literature review ......................................................... 488 8.3 The Zambezi River basin ............................................ 489 8.4 The Molluscan fauna .................................................. 491 8.5 Biogeography ............................................................... 508 8.6 Biomphalaria, Bulinis and Schistosomiasis ................ 515 8.7 Conservation ................................................................ 516 8.8 Further investigations ................................................. -

Lake Tanganyika, Regional Fisheries Programme (TREFIP)
FAO/NORWAY GOVERNMENT GCP/INT/648/NOR COOPERATIVE PROGRAMME Field Report F-14 (En) eries FISHCODE MANAGEMENT LAKE TANGANYIKA REGIONAL FISHERIES PROGRAMME (TREFIP) PREPARED BY THE JOINT AfDB/FAO/FISHCODE MISSION C. MAGNET, J.E. REYNOLDS AND H. BRU FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS ROME, JULY 2000 FAO/Norway Programme of Assistance to Developing Countries for the Implementation of the Code of Conduct for Responsible of the Code Conduct FAO/NorwayFish Programme of Assistance to Developing Countries for the Implementation Fisheries Management for the Provision Advice of Scientific for Improving Countries to Developing Assistance F: Sub-programme LAKE TANGANYIKA REGIONAL FISHERIES PROGRAMME (TREFIP) A proposal for implementation of the Lake Tanganyika Framework Fisheries Management Plan Prepared by: The Joint AfDB/FAO/FISHCODE Lake Tanganyika Mission Christophe Magnet (Team Leader/Economist, AfDB), J.Eric Reynolds (Development Planner/Socio-Economist, FAO), & Hervé Bru (Infrastructure/Marketing Specialist, AfDB) African Development Bank, Food and Agriculture Organization Abidjan of the United Nations, Rome July 2000 The designations employed and the presentation of material in this publication do not imply the expression of any opinion whatsoever on the part of the Food and Agriculture Organization of the United Nations concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. LAKE TANGANYIKA REGIONAL FISHERIES PROGRAMME (TREFIP) 18.07.00 ACKNOWLEDGEMENTS This document was drafted on behalf of the AfDB and the four Lake Tanganyika littoral States of Burundi, the Democratic Republic of Congo (DRC), Tanzania, and Zambia. Responsibility for its preparation was assigned to the Fisheries Policy and Planning Service (FIPP) of FAO, with funding provided by the AfDB and the FAO FISHCODE Programme (GCP/INT/648/NOR -- Interregional Programme of Assistance to Developing Countries for the Implementation of the Code of Conduct for Responsible Fisheries). -

Pangani Basin: a Situation Analysis
Pangani Basin: A Situation Analysis IUCN Eastern Africa Programme 2003 i Published by: Copyright: © 2003 International Union for Conservation of Nature and Natural Resources This publication may be produced in whole or part and in any form for education or non-profit uses, without special permission from the copyright holder, provided acknowledgement of the source is made. IUCN would appreciate receiving a copy of any publication which uses this publication as a source. No use of this publication may be made for resale or other commercial purpose without the prior written permission of IUCN. Citation: IUCN Eastern Africa Programme, 2003. The Pangani River Basin: A Situation Analysis, xvi + 104pp. ISBN: 2-8317-0760-9 Design and layout: Gordon O. Arara Printed by: ScanHouse Press Ltd. Photo 1: The summit of Mount Kilimanjaro; Photo 2: Forest stand at 1 Shire Njoro; Photo 3: Gate controlling the release of water into irrigation furrows; Photo 4: Children swimming in an irrigation 3 4 reservoir; Photo 5: Sisal plantations; Photo 6: Irrigated rice scheme; 2 Photo 7: Water gauging station at Chemka Spring; Photo 8: Vandalized gate controlling the release of water into irrigation furrows; Photo 9: 5 Dam wall at Nyumba ya Mungu Reservoir (color changes mark the declining water levels); Photo 10: A vendor sells water from a borehole 6 9 10 Photos 1, 3, 5, 6, 8, 9 copyright 2003 Kelly West; Photos 2, 7 7 8 copyright 2002 Kim Geheb; Photos 4, 10 copyright 2003 Ger Bergkamp. Available from: IUCN- EARO Publications Service Unit, P. O. Box 68200 - 00200, Nairobi, Kenya; Telephone ++ 254 20 890605-12; Fax ++ 254 20 890615; E-mail: [email protected] The designations of geographical entities in this book, and the presentation of the material, do not imply the expression of any opinion whatsoever on the part of the participating organiza- tions concerning the legal status of any country, territory, or area, or of its authorities, or con- cerning the delimitation of its frontiers or boundaries. -

The Cyphomyrus Myers 1960 (Osteoglossiformes: Mormyridae) of the Lufira Basin (Upper Lualaba: DR Congo): a Generic Reassignment and the Description of a New Species
Received: 9 January 2019 Accepted: 15 December 2019 DOI: 10.1111/jfb.14237 SPECIAL ISSUE REGULAR PAPER FISH The Cyphomyrus Myers 1960 (Osteoglossiformes: Mormyridae) of the Lufira basin (Upper Lualaba: DR Congo): A generic reassignment and the description of a new species Christian Mukweze Mulelenu1,2,3,4 | Bauchet Katemo Manda2,3,4 | Eva Decru3,4 | Auguste Chocha Manda2 | Emmanuel Vreven3,4 1Département de Zootechnie, Faculté des Sciences Agronomiques, Université de Abstract Kolwezi, Kolwezi, Democratic Republic of the Within a comparative morphological framework, Hippopotamyrus aelsbroecki, only Congo known from the holotype originating from Lubumbashi, most probably the Lubumbashi 2Département de Gestion des Ressources Naturelles Renouvelables, Unité de recherche River, a left bank subaffluent of the Luapula River, is reallocated to the genus en Biodiversité et Exploitation durable des Cyphomyrus. This transfer is motivated by the fact that H. aelsbroecki possesses a Zones Humides, Université de Lubumbashi, Lubumbashi, Democratic Republic of the rounded or vaulted predorsal profile, an insertion of the dorsal fin far anterior to the Congo level of the insertion of the anal fin, and a compact, laterally compressed and deep 3Vertebrate Section, Ichthyology, Royal Museum for Central Africa, Tervuren, Belgium body. In addition, a new species of Cyphomyrus is described from the Lufira basin, 4Laboratory of Biodiversity and Evolutionary Cyphomyrus lufirae. Cyphomyrus lufirae was collected in large parts of the Middle Lufira, Genomics, KU Leuven, Leuven, Belgium upstream of the Kyubo Falls and just downstream of these falls in the lower Lufira and Correspondence its nearby left bank affluent, the Luvilombo River. The new species is distinguished Emmanuel Vreven, Curator of Fishes from all its congeners, that is, firstly, from C. -

List of Rivers of Tanzania
Sl.No Name Draining Into 1 Bubu River Endorheic basins 2 Deho River East Coast 3 Great Ruaha River East Coast 4 Ifume River Congo Basin 5 Ipera River East Coast 6 Isanga River Nile basin 7 Jipe Ruvu River East Coast 8 Kagera River Nile basin 9 Kalambo River Congo Basin 10 Kavuu River Endorheic basins 11 Kihansi East Coast 12 Kikafu River East Coast 13 Kikuletwa River East Coast 14 Kimani River East Coast 15 Kimbi River East Coast 16 Kiseru River East Coast 17 Kizigo River East Coast 18 Kolungazao River East Coast 19 Lake Burunge Endorheic basins 20 Lake Eyasi Endorheic basins 21 Lake Jipe East Coast 22 Lake Malawi Zambezi basin 23 Lake Manyara Endorheic basins 24 Lake Natron Endorheic basins 25 Lake Rukwa Endorheic basins 26 Lake Tanganyika Congo Basin 27 Lake Victoria Nile basin 28 Little Ruaha River East Coast 29 Loasi River Congo Basin 30 Luamfi River Congo Basin 31 Luega River Congo Basin 32 Luegele River Congo Basin 33 Luengera River East Coast 34 Luhombero River East Coast 35 Lukigura River East Coast 36 Lukosi River East Coast 37 Lukuledi River East Coast 38 Lukumbule River East Coast 39 Lukwika River East Coast 40 Lungonya River East Coast 41 Luwegu River East Coast 42 Malagarasi River Congo Basin 43 Mara River Nile basin 44 Matandu River East Coast 45 Mavuji River East Coast 46 Mbarali River East Coast 47 Mbiki River East Coast 48 Mbungu River East Coast 49 Mbwemkuru River East Coast www.downloadexcelfiles.com 50 Mgeta River East Coast 51 Migasi River East Coast 52 Miyombo River East Coast 53 Mkata River East Coast 54 Mkomazi -

BREAK-OUT SESSIONS at a GLANCE THURSDAY, 24 JULY, Afternoon Sessions
2008 Joint Meeting (JMIH), Montreal, Canada BREAK-OUT SESSIONS AT A GLANCE THURSDAY, 24 JULY, Afternoon Sessions ROOM Salon Drummond West & Center Salons A&B Salons 6&7 SESSION/ Fish Ecology I Herp Behavior Fish Morphology & Histology I SYMPOSIUM MODERATOR J Knouft M Whiting M Dean 1:30 PM M Whiting M Dean Can She-male Flat Lizards (Platysaurus broadleyi) use Micro-mechanics and material properties of the Multiple Signals to Deceive Male Rivals? tessellated skeleton of cartilaginous fishes 1:45 PM J Webb M Paulissen K Conway - GDM The interopercular-preopercular articulation: a novel Is prey detection mediated by the widened lateral line Variation In Spatial Learning Within And Between Two feature suggesting a close relationship between canal system in the Lake Malawi cichlid, Aulonocara Species Of North American Skinks Psilorhynchus and labeonin cyprinids (Ostariophysi: hansbaenchi? Cypriniformes) 2:00 PM I Dolinsek M Venesky D Adriaens Homing And Straying Following Experimental Effects of Batrachochytrium dendrobatidis infections on Biting for Blood: A Novel Jaw Mechanism in Translocation Of PIT Tagged Fishes larval foraging performance Haematophagous Candirú Catfish (Vandellia sp.) 2:15 PM Z Benzaken K Summers J Bagley - GDM Taxonomy, population genetics, and body shape The tale of the two shoals: How individual experience A Key Ecological Trait Drives the Evolution of Monogamy variation of Alabama spotted bass Micropterus influences shoal behaviour in a Peruvian Poison Frog punctulatus henshalli 2:30 PM M Pyron K Parris L Chapman -

New Species of Congoglanis (Siluriformes: Amphiliidae) from the Southern Congo River Basin
New Species of Congoglanis (Siluriformes: Amphiliidae) from the Southern Congo River Basin Richard P. Vari1, Carl J. Ferraris, Jr.2, and Paul H. Skelton3 Copeia 2012, No. 4, 626–630 New Species of Congoglanis (Siluriformes: Amphiliidae) from the Southern Congo River Basin Richard P. Vari1, Carl J. Ferraris, Jr.2, and Paul H. Skelton3 A new species of catfish of the subfamily Doumeinae, of the African family Amphiliidae, was discovered from the Kasai River system in northeastern Angola and given the name Congoglanis howesi. The new species exhibits a combination of proportional body measurements that readily distinguishes it from all congeners. This brings to four the number of species of Congoglanis, all of which are endemic to the Congo River basin. ECENT analyses of catfishes of the subfamily Congoglanis howesi, new species Doumeinae of the African family Amphiliidae Figures 1, 2; Table 1 R documented that the species-level diversity and Doumea alula, Poll, 1967:265, fig. 126 [in part, samples from morphological variation of some components of the Angola, Luachimo River, Luachimo rapids; habitat infor- subfamily were dramatically higher than previously mation; indigenous names]. suspected (Ferraris et al., 2010, 2011). One noteworthy discovery was that what had been thought to be Doumea Holotype.—MRAC 162332, 81 mm SL, Angola, Lunda Norte, alula not only encompassed three species, but also that Kasai River basin, Luachimo River, Luachimo rapids, 7u219S, they all lacked some characters considered diagnostic of 20u509E, in residual pools downstream of dam, A. de Barros the Doumeinae. Ferraris et al. (2011) assigned those Machado, E. Luna de Carvalho, and local fishers, 10 Congoglanis species to a new genus, , which they hypoth- February 1957. -

October 29, 2019 Tanzania Electric Supply Company Limited
ENVIRONMENTAL AND SOCIAL IMPACT ASSESSMENT SUMMARY FOR THE PROPOSED CONSTRUCTION OF 44.8MW MALAGARASI HPP AND ASSOCIATED 132KV TRANSMISSION LINE FROM MALAGARASI HYDROPOWER PLANT TO KIGOMA 400/132/33KV SUBSTATION AT KIDAHWE KIGOMA OCTOBER 29, 2019 TANZANIA ELECTRIC SUPPLY COMPANY LIMITED 1 PROJECT TITLE: MALAGARASI 45MW HYDRO POWER PROJECT PROJECT NUMBER: P-TZ-FAB-004 COUNTRY: TANZANIA CATEGORY: 1 Sector: PICU Project Category: 1 2 1. TABLE CONTENTS 1. TABLE CONTENTS ............................................................................................................................................................... 3 2. INTRODUCTION.................................................................................................................................................................. 4 3. PROJECT DESCRIPTION ....................................................................................................................................................... 4 4. PROJECT DESCRIPTION ....................................................................................................................................................... 6 5. POLICY AND LEGAL FRAMEWORK ....................................................................................................................................... 6 6. ENVIRONMENTAL AND SOCIAL BASELINE ............................................................................................................................ 7 7. STAKEHOLDER ENGAGEMENT PROCESS .............................................................................................................................