Changes in Selected Elements of Soils Under Simulated Acid Rain Conditions
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

5BU Doc Catalog 3-15 Layout 1
he faculty members of the Doctoral program L. Francesca Ferrari have been carefully selected from the many L.Ac. Faculty renowned Traditional Chinese Medicine DMQ (China), Western District Medical Qigong Science and T TCM Research Institute, Beijing, China (TCM) teachers in the United States, China, and Europe. Many of our faculty descend from a lineage of DTCM (China), Ministry of Health, China TCM Masters in China and have over 40 years experi- Instructor, Five Branches University, Santa Cruz, California Associate Dean, Medical Qigong Science, Five Branches ence practicing and teaching TCM. Several have University, Santa Cruz, California trained in China’s most prestigious TCM universities Associate Director, Medical Qigong Clinic, Five Branches and hospitals, such as Shanghai University of TCM, University, Santa Cruz, California Beijing University of Chinese Medicine, Guangzhou Overseas Faculty (Dean of TCM), Medical Qigong College, Henan University of TCM, China University of TCM, Liaoning University of TCM, Traditional Chinese Medical Qigong Tianjin University of TCM, Fujian University of DAOM TCM, and Zhejiang Chinese Medical University. Nadine Gassner DAOM faculty also includes seasoned medical doctors Ph.D. who teach Western Medicine classes. Most of our PhD, Chemistry, University of Oregon, Eugene instructors are fluent in English; Chinese speaking fac- Former Research Associate, Stanford University School of ulty are accompanied by competent translators. All of Medicine, California our DAOM faculty are experienced and successful, Former Research Associate, Yale University School of Medicine, teacher-practitioners, and demonstrate a dedication to Connecticut The TCM and a passion for healing. Research Associate, University of California, Santa Cruz Director of Research, Five Branches University, Santa Cruz, California U.S. -

2021 International Conference on Security, Pattern Analysis, and Cybernetics (SPAC 2021)
2021 International Conference on Security, Pattern Analysis, and Cybernetics (SPAC 2021) Honorary General Chairs Tianyou Chai, Chinese Academy of Sci., China Jie Chen, Beijing Institute of Technology, China General Chair C. L. Philip Chen, South China Univ. of Tech., China General Co-Chair Yuhua Cheng, University of Electronic Science and Technology of China, China Weidong Zhang, Shanghai Jiao Tong University, China Program Chair Tieshan Li, University of Electronic Science and Technology of China Program Co-Chairs Jinliang Shao, University of Electronic Science and Technology of China, China Yuan-Yan Tang, University of Macau, Macau, China CALL FOR PAPERS: Xiaofeng Liao, Chongqing University, China Songyi Dian, Sichuan University, China 2021 International Conference on Security, Pattern Analysis, and Cybernetics Lu Liu, City University of Hong Kong, HK, China June 18-20, 2021 Organizing Committee Chairs Hong Cheng, University of Elect. Sci. and Tech. of University of Electronic Science and Technology of China, Chengdu, Sichuan, China, China China. Dujuan Wang, Sichuan University, China Yue Long, University of Electronic Science and Location: Technology of China, China Yan-Jun Liu, Liaoning University of Tech., China The 2021 International Conference on Security, Pattern Analysis, and Cybernetics will be held by University Yong-Ming Li, Liaoning University of Tech., China of Electronic Science and Technology of China, Chengdu, Sichuan, China. Organizing Committee Co-Chairs Sponsors: Kai Chen, University of Electronic Science and Sponsors: -
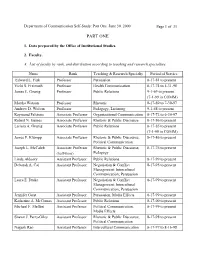
Self-Study Part I
Department of Communication Self-Study: Part One June 30, 2000 Page 1 of 31 PART ONE 1. Data prepared by the Office of Institutional Studies. 2. Faculty. A. List of faculty by rank, and distribution according to teaching and research specialties. Name Rank Teaching & Research Specialty Period of Service Edward L. Fink Professor Persuasion 8-17-81 to present Vicki S. Freimuth Professor Health Communication 8-17-74 to 5-31-98 James E. Grunig Professor Public Relations 9-1-69 to present (7-1-99 in COMM) Martha Watson Professor Rhetoric 8-17-89 to 7-30-97 Andrew D. Wolvin Professor Pedagogy, Listening 9-1-68 to present Raymond Falcione Associate Professor Organizational Communication 8-17-72 to 6-30-97 Robert N. Gaines Associate Professor Rhetoric & Public Discourse 8-17-86 to present Larissa A. Grunig Associate Professor Public Relations 8-17-85 to present (7-1-99 in COMM) James F. Klumpp Associate Professor Rhetoric & Public Discourse, 8-17-86 to present Political Communication Joseph L. McCaleb Associate Professor Rhetoric & Public Discourse, 8-17-76 to present (half-time) Pedagogy Linda Aldoory Assistant Professor Public Relations 8-17-99 to present Deborah A. Cai Assistant Professor Negotiation & Conflict 8-17-95 to present Management; Intercultural Communication; Persuasion Laura E. Drake Assistant Professor Negotiation & Conflict 8-17-99 to present Management; Intercultural Communication; Persuasion Jennifer Garst Assistant Professor Persuasion, Media Effects 8-17-99 to present Katherine A. McComas Assistant Professor Public Relations 8-17-00 to present Michael F. Meffert Assistant Professor Political Communication, 8-17-99 to present Media Effects Shawn J. -

8Th IWACP Participants
Hosted by Guangdong University of Technology, Beijing Normal University, Hainan Tropical Ocean University Organized by Co-organized by Paulista University, Dongguan University of Technology, Parthenope University, University of Sheffield, Deakin University in participation with 2 8th International Workshop | Advances in Cleaner Production Technical Report Responsible for the information: Biagio F. Giannetti (Conference Chair) http://www.advancesincleanerproduction.net COUPLING GREEN AND BLUE ECONOMIES: HOW ARE CLEANER PRODUCTION AND CITIES LEADING THE NEXT SUSTAINABLE DEVELOPMENT? Sanya, China November 13th to 15th - 2019 th 3 8 International Workshop | Advances in Cleaner Production The topic of the 8th International Workshop Advances in Cleaner Production (IWACP): Coupling green and Blue Economies: How are Cleaner Production and Cities Leading the Next Sustainable Development? was discussed by Brazilian and foreign researchers for three days in Sanya, China during the period of November 13th to 15th, 2019. The international conference was organized by the Postgraduate Program in Production Engineering (Master's and Doctoral Program) and Co-organized by Dongguan University of Technology, Parthenope University, University of Sheffield, Deakin University. The event counted with the partnership of the (University of Firenze, Italy), UNISON (Universidad de Sonora, México), the University of Manitoba (Canada), BNU (Beijing Normal University, China), the Journal of Cleaner Production, and the ACPN (Advances in Cleaner Production Network). The hosting institutions were the Guangdong University of Technology, Beijing Normal University, and Hainan Tropical Ocean University The partnership with the Journal of Cleaner Production (https://www.journals.elsevier.com/journal-of-cleaner-production/), that started during the 1st IWACP was maintained with the support of the Co-Editors in Chief: Prof. -

The Relationship Between the Facial Expression of People in University Campus and Host- City Variables
Supplementary: The relationship between the facial expression of people in university campus and Host- city variables Hongxu Wei 1, Richard J. Hauer 2 and Xuquan Zhai 3,* 1 Northeast Institute of Geography and Agroecology, Chinese Academy of Sciences, Changchun 130102, China; [email protected] 2 College of Natural Resources, University of Wisconsin–Stevens Point, 800 Reserve St., Stevens Point, WI 54481, United States; [email protected] 3 China Center for Public Sector Economy Research, Jilin University, Room 3007, Kuang, Yaming Building, 2699 Qianjin Road, Chaoyang District, Changchun 130012, China, Changchun 130012, China; * Correspondence: [email protected]; Tel.: +86-431-8516-8829 Figure S1. The panel of FireFACETM-V1.0 to recognize photos with typically happy, sad, and neutral facial expressions. The model is Ryōko Hirosue from Japan. Photos are downloaded from following websites: Happy: https://images.app.goo.gl/Kh5FgWEMMSPUa3sD9 Sad: https://images.app.goo.gl/GazwjvTh8a6qiyBa9 Neutral: https://images.app.goo.gl/Hn2gtLonVpoLd5o27 Figure S2. The copyright of the FireFACETM-V1.0 software that is authorized in mainland China. Table S1. The list of key universities in the 211-Project of mainland China with Province and City names. Rank Province City University name 1 Anhui University 2 Anhui Hefei Hefei University of Technology 3 University of Science and Technology of China 4 Beijing Foreign Studies University 5 Beijing Forestry University 6 Beijing Institute of Technology 7 Beijing Jiaotong University 8 Beijing Normal University -

Initiative Disciplines Development List 01 Natural and Physical Sciences (100)
“Double First-Class” initiative disciplines development list (Sorted by discipline) Note: The list was published by the Ministry of Education of the People’s Republic of China on 21 September 2017. This list sorted by discipline has been prepared by the Education and Research Section, Australian Embassy, Beijing based on the Australian Standard Classification of Education (ASCED) 2001, Australian Bureau of Statistics. Initial translation credit to Science, Technology and Education Section, Embassy of Switzerland in China. 01 Natural and Physical Sciences (100) Astronomy (2) Nanjing University University of Science and Technology of China Atmosphere Science (3) Nanjing University Nanjing University of Information Science & Technology Lanzhou University Biology (16) Peking University Tsinghua University China Agricultural University Peking Union Medical College Inner Mongolia University Fudan University Shanghai Jiao Tong University Nanjing University Zhejiang University University of Science and Technology of China Xiamen University Huazhong Agricultural University Henan University Sun Yat-sen University Wuhan University Southwest University Chemistry (25) Peking University Tsinghua University Nankai University Tianjin University Dalian University of Technology Jilin University Northeast Normal University Fudan University Shanghai Jiao Tong University East China University of Science and Technology Nanjing University Zhejiang University Xiamen University University of Science and Technology of China Fuzhou University Shandong University -

Committee List
ICNSC 2015 Committee List General Chair Prof. Zhihong Qian, Jilin University, Changchun, Jilin, China Honor Chair Prof. Jianquan Ouyang, College of information engineering Xiangtan University, China Prof. Hongwei GE, Dalian University of Technology, China Co-Chair Associate Professor, Hong LU, Fudan University, China Associate Professor, Qiang Liu, University of Electronic Science and Technology of China, China Associate Professor, Shaohui Liu, Harbin Institute of Technology, China The Advisory Committee Associate Professor, Feng Wang, Wenzhou University, China Associate Professor, Lingtao Zhang, Central South University of Forestry and Technology, China The Science Committee Prof. Weijin Jiang, Hunan Business College, China Associate Professor, Hongzhi Wang, Harbin Institute of Technology, China Associate Professor, Yinghua Zhang, Chinese Academy of Sciences, China Dr. Bo Li, Liaoning University of Technology, China The Organizing Committee Dr. Lynn Wang, CNIS2015 Organizer Committee Dr. Xianzhi Wang, Harbin Institute of Technology, China Technical Program Committee Prof. Jianquan Ouyang, College of information engineering Xiangtan University, China Senior engineer, Dong Huang, a visiting fellow (visiting professor) of Chinese Academy of Sciences, China Prof. Hongwei GE, Dalian University of Technology, China Assistant Professor, Hong LU, Fudan University, China Associate Professor, Qiang Liu, University of Electronic Science and Technology of China, China Associate Professor, Shaohui Liu, Harbin Institute of Technology, China Associate Professor, Feng Wang, Wenzhou University, China xvi Associate Professor, Lingtao Zhang, Central South University of Forestry and Technology, China Prof. Weijin Jiang, Hunan Business College, China Associate Professor, Hongzhi Wang, Harbin Institute of Technology, China Assistant Professor, Yinghua Zhang, Chinese Academy of Sciences, China Dr. Bo Li, Liaoning University of Technology, China Dr. Lynn Wang, CNIS2015 Organizer Committee Dr. -

Majors of Liaoning University
Liaoning University Introduction In the course of over half a century’s development, Liaoning University has grown into a multiple-discipline comprehensive university, embracing Chinese language and literature, history, philosophy, economics, law, foreign languages, art, natural science, engineering and management. Liaoning University is one of the key universities listed in the nation’s “Project 211” and affirmed by the Ministry of Education. The University has 25 colleges, which offer the whole 62 majors of undergraduate disciplines, 160 master-degree-granting disciplines and the university is also invested with authority to grant 62 doctorates. Since 1965, Liaoning University has been admitting foreign students from over 94 countries, such as U.S.A., Japan, Russia, Korea, Italy, England, France, etc. Over 8000 long-term students and 3000 short-term students enjoyed their study in Liaoning University. The number of foreign students now on campus is approaching 700. Liaoning University welcomes the students from all over the world to study here. Degree Programs Bachelor’s degree (4 years) General Scholar (1-2years) 1. Chinese Language (Only for International students) 2. Chinese Language and Literature 3. Advertisement 4. Journalism 5. History 6. Archives 7. Philosophy 8. Administration 9. Labor and Social Security 10. Economics 11. National Economic Administration 12. Statistics 13. Public Finance 14. Management of Engineering 15. Finance 16. Insurance 17. International Economics and Trade 18. International politics 19. Accounting 20. Marketing 21. Business Administration 22. Tourism Management 23. Science of Law 24. Broadcasting and TV Editing and Directing 25. Broadcast and Television Journalism 26. Pure and Applied Mathematics 27. Information and Computational Science 28. -

Agency Number – Name of the University/Embassy
Agency Number – Name of the University/Embassy 10001 – Peking University 10002 – RENMIN UNIVERSITY OF CHINA 10003 – TSINGHUA UNIVERSITY 10004 – BEIJING JIAOTONG UNIVERSITY 10005 – BEIJING UNIVERSITY OF TECHNOLOGY 10006 – Beihang Univ. (BUAA) 10007 – Beijing Institute of Technology 10008 – Univ. of Science and Technology Beijing 10010 – Beijing Univ. of Chemical Tech. 10013 – Beijing Univ. of Posts and Telecommunications 10019 – China Agricultural Univ (CAU) 10022 – Beijing Forestry Univ. 10026 – Beijing University of Chinese Medicine 10027 – Beijing Normal Univ. 10028 – Capital Normal Univ. 10029 – Capital Institute of Physical Educations 10030 – Beijing Foreign Studies University 10031 – Beijing International Studies Univ. 10032 – Beijing Language and Culture Univ. 10034 – Central Univ. of Finance and Economics 10036 – Univ. of International Business and Economics 10038 – Capital Univ. of Business and Economics 10040 – China Foreign Affairs Univ. 10043 – Beijing Sport University 10045 – Central Conservatory of Music 10047 – Central Academy of Fine Arts 10048 – The Central Academy of Drama 10050 – Beijing Film Academy 10052 – Central University For Nationalities 10053 – China University of Political Science and Law 10054 – North China Electric Power University 10055 – Nankai University 10056 – Tianjin Univ. 10057 – Tianjin Univ. of Science and Technology 10062 – Tianjin Medical University 10063 – Tianjin Univ. of Traditional Chinese Medicine 10065 – Tianjin Normal Univ 10066 – Tianjin Univ. of Technology and Education 10068 – Tianjin Foreign Studies Univ. (TFSU) 10140 – Liaoning University 10141 – Dalian Univ. of Technology 10145 – Northeastern University 10151 – Dalian Maritime Univ. 10159 – China Medical University 10161 – Dalian Medical University 10165 – Liaoning Normal University 10166 – Shenyang Normal University 10172 – Dalian Univ. of Foreign Languages 10173 – Dongbei Univ of Finance and Economics 10183 – Jilin University 10184 – Yanbian Univeristy 10186 – Changchun Univ. -
Corruption and Irregularities in Academic Activities and Abnormal Academic Ethos
CORRUPTION AND IRREGULARITIES IN ACADEMIC ACTIVITIES AND ABNORMAL ACADEMIC ETHOS Ge Jianxiong In recent years, academic corruption has become more and more seri- ous in China, while academic irregularities and an abnormal academic ethos have also gone rampant on an unprecedented scale. According to a survey regarding the situation of scientific and technical workers across the country published by the China Society and Technology Association in July 2007, almost half of scientific and technical work- ers deemed academic irregularities as a universal phenomenon, and more than half of them stated clearly that this behavior was common among the researchers around them.1 In February 2009, a thesis fraud scandal involving He Haibo, a post-doctoral student from the College of Pharmaceutical Sciences of Zhejiang University, was exposed by the media. A series of aca- demic papers published in international leading academic journals were recalled after He, the first author of such papers, was found to have been involved in improper practices, such as fabricating data and sending papers to different journals for publication. Other authors included key members of He’s research group and Li Lianda, a mem- ber of the Chinese Academy of Engineering and the dean of the university’s College of Pharmaceutical Sciences.2 In mid-June, What is “theory”, an article published in a well-known national journal of philosophy and co-authored by Lu Rongjie, the vice president of Lia- oning University and Yang Lun, a doctoral student at Beijing Normal University, was proved to be an 80% copy of lecture material prepared by Wang Lingyun, a lecturer at Yunnan University.3 At the end of June, it was reported that seven postgraduate students with scholarship 1 Sun Zifa, “The 2nd Survey Report on the Situation of National Scientific and Technical Workers,” China News Net, July 10, 2007. -
Chinese Student Contact List (Updated: April, 2020)
Chinese Student Contact List (updated: April, 2020) The students listed below are from China in the Price School of Public Policy. They are willing to have prospective students contact them about their experiences in the program and their experiences acclimating to USC, Los Angeles, and the United States. Please note that the Price School revised the Master of Planning (MPL) degree in 2019. The program is now called Master of Urban Planning (MUP). Si Chen Yuxiao (Ada) Han Master of Public Policy (MPP) Master of Public Administration (MPA) Zhejiang University Sichuan International Studies University (English) (Food Science and Engineering) Harbin, Heilongjiang Ningbo [email protected] [email protected] Yingshi Huang Qinhui Fang Master of Planning (MPL) Master of Public Policy (MPP) South China University of Technology Huazhong University of Science and Technology (Urban Planning) (Sociology) Guangzhou Hangzhou [email protected] [email protected] Yifan Jiang Yiqing Feng Master of Public Policy (MPP) Master of Public Administration (MPA) University of California, Santa Barbara Beijing Technology and Business University (Global Studies) (Economics) Hefei/Anhui Taiyuan, Shanxi [email protected] [email protected] Chengzhao Li Yinan Gao Master of Public Policy (MPP) Master of Planning (MPL) University of International Relationships University of California, Santa Barbara (International Politics) (Environmental Studies) Anhui Shaanxi [email protected] [email protected] Yuqiao Gao Dongyang Lin Master of Public Administration (MPA) Master of Urban Planning -

Data Sources
DATA SOURCES Most of the data used in this report come from statistical yearbooks. These statisti- cal yearbooks are compiled by relevant authorities in charge, usually on an annual basis. Therefore, they represent the authoritative source of information. The most comprehensive yearbooks are the China Statistical Yearbook (Series), compiled by the National Bureau of Statistics. These yearbooks cover many signifi- cant national statistics, relavent to the education system and science and technology developments. The China Education Yearbook (Series) is compiled by the Ministry of Ed- ucation and provides more detailed statistics on education. The China Labour Statistical Yearbook (Series) and China Statistical Yearbook of Science and Tech- nology (Series), compiled with input from the Ministry of Human Resources and Social Security and the Ministry of Science and Technology, also include data relevant to higher education and higher education out puts. Other more specific yearbooks, such as the China Educational Finance Sta- tistical Yearbook (Series) provides detailed and specific statistics in focused areas. Below is a list of the yearbook sources: Educational Statistics Yearbook of China (Series), Author: Department of Development and Planning, Ministry of Education, People’s Republic of China Publisher: Peoples’ Education Press China Education Yearbook (Series), Author: Ministry of Education, People’s Republic of China Publisher: China Education Press China Statistical Yearbook (Series), Author: National Bureau of Statistics of China Publisher: