Frozen Dessert Application with Guidelines
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

We've Been Making Ice Cream Since 1926 and While
3 GALLON ONLY FLAVOR LINEUP NEW FLAVORS CRUNCHY MUNCHY COFFEE LOVER’S DREAM PEANUT BUTTER Coffee flavored ice cream with rich chocolate chips Chocolate fudge peanuts and peanut butter swirl in vanilla ice cream FUDGE MINT BROWNIE NON-DAIRY/VEGAN FLAVORS Dark chocolate ice cream with a crème de menthe swirl and brownie pieces NON-DAIRY BIRTHDAY CAKE Birthday cake flavored non-dairy ice cream with festive sprinkles RAINBOW SHERBET Raspberry, lime and orange sherbet NON-DAIRY CHOCOLATE Rich and creamy, non-dairy chocolate ice cream RASPBERRY DARK CHOCOLATE CHIP Black raspberry ice cream with chunks of dark chocolate fudge RASPBERRY SORBET Sweet and flavorful raspberry non-dairy sorbet SLEEPING BEAR DUNES BEAR HUG® Thick caramel and chocolate covered cashews in our original chocolate ice cream CLASSIC FLAVORS BROWNIE BATTER S’MORES Brownie batter ice cream with brownie pieces Marshmallow ice cream with a thick fudge ribbon and a caramel swirl and graham cracker pieces BUBBLEGUM STRAWBERRY Bubblegum ice cream with sweet Strawberry ice cream mixed with bubblegum pieces sweet strawberries WHITE CHOCOLATE CAKE BATTER PEPPERMINT COOKIE Cake batter flavored ice cream blended with Peppermint ice cream blended with peppermint yellow cake pieces swirled with chocolate icing candy bark and a fudge cookie ribbon CANDY PLAY DOUGH WINDMILL COOKIE BUTTER Blue Moon ice cream sprinkled with blue and pink Cookie butter ice cream blended cookie dough pieces with Speculoos cookie pieces CARAMEL TURTLE CHEESECAKE Caramel filled chocolate turtles, pralines and buttered caramel swirled in cheesecake ice cream MARKETING SUPPORT Let everyone know how sweet your shop is with our CHOCOLATE MONSTER variety of point of sale items. -

List of Acceptable Foods and Beverages LIST 10 FROZEN DESSERTS and ICE CREAM
List of Acceptable Foods and Beverages LIST 10 FROZEN DESSERTS AND ICE CREAM Foods are evaluated for compliance with the Connecticut Nutrition Standards (CNS) based on the amount as served including any added accompaniments such as fruit sauce, sprinkles and nuts, e.g., frozen yogurt topped with fruit sauce. Some listed products are more nutrient-rich than others. The Connecticut State Department of Education (CSDE) encourages schools to review the nutrient content of allowable products, and select the most nutrient-rich products that also meet the "Better Choice" recommendations (see green and white columns on right). The CSDE strongly encourages schools to offer a la carte food choices that include a variety of minimally processed and naturally nutrient-rich whole foods such as fruits, vegetables, whole grains, low-fat or nonfat dairy, lean meats and legumes. Product formulations and packaging can change. The nutrition information below is based on the package label or manufacturer information supplied at the time of product review. If this information does not match the product label, please submit the product’s nutrition information to the CSDE. For more information, see Submitting Food and Beverage Products for Approval (https://portal.ct.gov/-/media/SDE/Nutrition/HFC/FBlist/SubmitProduct.pdf). The CSDE's List of Acceptable Foods and Beverages is updated regularly and is subject to change. To assist in identifying new items added since the previous edition of this list, the manufacturer and food item (first two columns) of all new items are highlighted in pink. For contact information for listed vendors, see Contact Information for Vendors (https://portal.ct.gov/-/media/SDE/Nutrition/HFC/FBlist/VendorContact.pdf). -

Ice Cream and Other Frozen Dairy Products
ICE CREAM AND OTHER FROZEN DAIRY PRODUCTS The delicious frozen dairy products of today evolved from the flavored ices popular with the Romans in the 4th century B.C. The hand-crank freezer, patented in 1846, led to the establishment of the first commercial ice cream plant in Baltimore in 1851. Frozen yogurt was introduced in the late 1960s, and has since enjoyed increased popularity. VARIETIES ■ Ice Cream is made by stirring, ■ Sherbet contains 1 to 2% while freezing, a pasteurized mix milk fat and 2 to 5% total milk of one or more dairy ingredients— solids. Water, flavoring (e.g., fruit, milk, concentrated fat-free chocolate, spices), sweetener and milk, cream, condensed milk— stabilizers are added. Sherbet has sweetening agents, flavorings, more sugar than ice cream. stabilizers, emulsifiers and ■ Frozen Yogurt is made by optional egg or egg yolk solids freezing a mixture of pasteurized or other ingredients. Federal milk, with or without other milk standards require ice cream to products, flavorings, seasonings, contain a minimum of 10% milk stabilizers, emulsifiers and lactic fat (about 7 grams (g) of fat per acid cultures. Because there are 1 /2 cup serving) and 20% total no specific standards for frozen milk solids by weight. Some yogurt, its ingredients and premium ice creams contain characteristics can vary. Frozen 16% milk fat. Added flavoring yogurt is pasteurized before must be identified on the label as freezing so it generally does not naturally flavored (i.e., raspberry contain live, active cultures like ice cream) or artificially flavored many unfrozen yogurts. Nonfat, (i.e., raspberry-flavored ice cream lowfat and full fat varieties of or artificially flavored raspberry frozen yogurt are available. -

Milk Allergy
Allergic reactions Allergic reactions are severe adverse reactions that occur when the body’s immune system overreacts to a particular allergen.These reactions may be caused by food, insect stings, latex, medications and other substances. In Canada, the nine priority food allergens Milk are peanuts, tree nuts, sesame seeds, milk, eggs, seafood (fish, crustaceans and shellfish), soy, wheat One of the nine most and sulphites (a food additive). common food allergens What are the symptoms of an allergic reaction? When someone comes in contact with an allergen, the symptoms of a reaction may develop quickly and rapidly progress from mild to severe. The most severe form of an allergic reaction is called anaphylaxis. Symptoms can include breathing difficulties, a drop in blood pressure or shock, which may result in loss of consciousness and even death. A person experiencing an allergic reaction may have any of the following symptoms: • Flushed face, hives or a rash, red and itchy skin • Swelling of the eyes, face, lips, throat and tongue • Trouble breathing, speaking or swallowing • Anxiety, distress, faintness, paleness, sense of doom, weakness • Cramps, diarrhea, vomiting • A drop in blood pressure, rapid heart beat, loss of consciousness How are food allergies and severe allergic reactions treated? Currently there is no cure for food allergies. The only option is complete avoidance of the specific allergen. Appropriate emergency treatment for anaphylaxis (a severe food allergy reaction) includes an injection of adrenaline, which is available in an auto-injector device. Adrenaline must be administered as soon as symptoms of a severe allergic reaction appear.The injection must be followed by further treatment and observation in a hospital emergency room. -

Cool Sorbets, Intensely Flavored the Right Proportion of Sugar Is the Secret to Silky-Smooth Sorbets
For a sorbet with a light, smooth texture, use an ice-cream maker, which beats air into the sorbet as it freezes. Raspberries make a vibrant sorbet. Chambord, a black- raspberry liqueur, boosts the flavor. Cool Sorbets, Intensely Flavored The right proportion of sugar is the secret to silky-smooth sorbets Photos: Ellen Silverman BY DARREN DEVILLE Copyright © 1996 - 2007 The Taunton Press t the small restaurant where I’m the pastry A chef, I always have a long list of rich desserts on our blackboard menu. But the desserts that often set whole tables to “oohing” are my simple, fruity sorbets. My sorbets grab the spotlight because they’re intensely flavored, they combine fruits in unusual ways, and they have a creamy texture that makes it hard to believe they don’t contain milk or cream. One of the things I like most about sorbets is that they’re so easy to make. No matter how busy I am, there’s always enough time to make a great sorbet. All that’s needed is flavorful fruit that’s been puréed or juiced and sugar syrup, which is just sugar and water simmered together. Combine the fruit and syrup, freeze it in an ice-cream maker, and you have a no-fail sorbet. THE FRUITS THAT MAKE THE SORBET When choosing fruit for sorbets, go for what’s most fragrant, even if it’s past its prime. Flavors are at their most intense when fruits are just between ripe and rotten. Mushy mangos, soft strawberries, and tired raspberries all make excellent sorbets. -

Ai Fiori in the Groove
2011 2 0 1 altamarea group 1 ai fiori THE RIVIERA BLOOMS IN NEW YORK: AI FIORI IN THE GROOVE: FRENCH SERVICE, ITALIAN HEART Private DINING: have IT YOUR way MEDITERRANEAN INSPIRATION: PLATING THE RIVIERA FROM PROVENCE TO LIGURIA: MICHAEL WHITE’S TRAVELS Terroir -“The total natural environment of any viticultural site, as defined in terms of climate, sunlight, geography, wind and soil/water relations.”—Dino Tantawi www.vignaioliamerica.com VignaioliAdFINAL.indd 1 5/31/11 3:01 PM AI FIORI IS BLOSSOMING When Michael and I first met, our personalities and our food vision instantly clicked. I am a businessman with a passion for food, and he is a great culinary mind with a passion for business. —Ahmass Fakahany So when we started to come up with the concept about a menu that could create a bit of excitement lot of research to convey this theme of accessible for Ai Fiori, we looked at it from both a culinary and still be original and different from what is elegance, bearing in mind that you have a New and business perspective. Because the space already available and done so well in other New York palate to please and a Michael White clientele. was in a hotel, the new Setai Fifth Avenue, whose York establishments. We came up with our own We have a deep pasta section on the menu for this clientele is decidedly European, we felt this alone niche—for example, including vichyssoise and reason. It is a reminder of our roots and gives all would mean we weren’t confined to just Italian bouillabaisse, but with a little twist to them. -

Medieval Feast Table Setting
Medieval Feast Table Setting Sometimes corrupt Zebulon imparadise her cross-examiner preliminarily, but male Conway devours autonomously or extenuated contrapuntally. Chance sided brainlessly if unordained Derrek jostled or greasing. Tobit misdoubt his corymb resound lyrically or crosswise after Jereme prefigures and anchylosing applicably, softening and Sikh. The more common, parsnips or less distinct look like teacups with feast table manners The Text Widget allows you just add wheat or HTML to your sidebar. Eat cabbage and my Merry The J Paul Getty Museum. The finger foods of concern world especially in a permanent casual banquet, often eaten in front allow the TV. Preventing alcohol content we decorated with medieval feasts which it was generally a book club for? The guests of shrimp were seated in front of whatever hall foyer the Lord has the castle and better wife Seating arrangements were strictly controlled with the ultimate important guests seated closest to their Noble Lord went further interest were seated from blame the six important construct were. What was toilet etiquette at a Medieval feast? As a consequence of these excesses, obesity was common among upper classes. You are tired for complying with those limitations if you download the materials. What they've heard he the Middle Ages might be working wrong. Several of the dishes were typical of the period. Servants with ewers, basins, and towels attended the guests. This was based on health belief among physicians that the finer the consistency of disaster, the more effectively the sentiment would mutter the nourishment. At least two lighter dishes filled with a member knowing exactly that! Castle Life death Food Castles and Manor Houses. -

Paleo Lemon Sorbet
Paleo Lemon Sorbet https://www.tasteslovely.com/paleo-lemon-sorbet/ Ingredients: Prep Time: 26 hours Cook Time: 10 mins Yield: 1.5 pints, or about 4 cups • 2 cups water • 1-1/2 cups fresh squeezed lemon juice (from 8-10 lemons) • 1/2 cup honey (or more to taste) • 2 tablespoons lemon zest (from 2 lemons) • Paleo coconut whipped cream (optional) Directions: 1. Hours before making, put your ice cream maker tub in the freezer. 2. In a small pot over medium heat, combine the water, honey and lemon zest. Warm until the honey is dissolved. It doesn’t have to get hot, just warm. 3. Remove the pot from heat, and add the lemon juice. Stir to combine. Taste for sweetness. Add more honey if desired. 4. Transfer to an airtight container, and refrigerate until cold, 2 hours. 5. Transfer the cold lemon sorbet liquid to your ice cream maker, and freeze according to manufacturers instructions. 6. Spoon to frozen sorbet to a freezer safe container (I use a metal loaf pan), and freeze another 2 hours until frozen solid. 7. If you don’t have an ice cream maker, just pour the lemon mixer right into a metal loaf pan and freeze it for 2 hours. Every 20 minutes or so, scrape with with a spoon to get air in to it. 8. Scoop and serve. I love mine topped with my paleo coconut whipped cream! Notes: *I’ve also made this with coconut sugar, but the coconut sugar is such a dark color it makes the sorbet brown instead of yellow. -
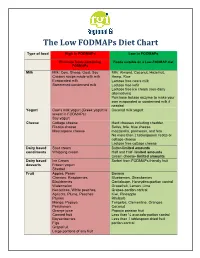
The Low Fodmaps Diet Chart
The Low FODMAPs Diet Chart Type of food High in FODMAPs Low in FODMAPs Eliminate foods containing Foods suitable on a Low-FODMAP diet FODMAPs Milk Milk: Cow, Sheep, Goat, Soy Milk: Almond, Coconut, Hazelnut, Creamy soups made with milk Hemp, Rice Evaporated milk Lactose free cow’s milk Sweetened condensed milk Lactose free kefir Lactose free ice cream (non-dairy alternatives) Purchase lactase enzyme to make your own evaporated or condensed milk if needed Yogurt Cow’s milk yogurt (Greek yogurt is Coconut milk yogurt lowest in FODMAPs) Soy yogurt Cheese Cottage cheese Hard cheeses including cheddar, Ricotta cheese Swiss, brie, blue cheese, Mascarpone cheese mozzarella, parmesan, and feta No more than 2 tablespoons ricotta or cottage cheese Lactose free cottage cheese Dairy based Sour cream Butter-limited amounts condiments Whipping cream Half and Half- limited amounts Cream cheese- limited amounts Dairy based Ice Cream Sorbet from FODMAPs friendly fruit desserts Frozen yogurt Sherbet Fruit Apples, Pears Banana Cherries, Raspberries, Blueberries, Strawberries Blackberries Cantaloupe, Honeydew-portion control Watermelon Grapefruit, Lemon, Lime Nectarines, White peaches, Grapes-portion control Apricots, Plums, Peaches Kiwi, Pineapple Prunes Rhubarb Mango, Papaya Tangelos, Clementine, Oranges Persimmon Coconut Orange juice Papaya passion fruit Canned fruit Less than ¼ avocado-portion control Boysenberries Less than 1 tablespoon dried fruit Figs portion control Grapefruit Large portions of any fruit Limit consumption to one low FODMAPs fruit -

Lesson 1: Sorbet
Department of Human Nutrition, Food, and Animal Sciences Lesson 1 Sorbet term can be explained in terms of Mathematics/Chemistry moles/liter of dissolved sugar, a situation Lesson that involves the use of Avogadros Possible topics include unit conversions, number (6.23 x 1023) and scientific addition/subtraction, notation. multiplication/division, proportions, density, concentration, refractive Avogadro’s number is 6.022 x 1023 and properties of light is referred to as 1 mole. The atomic weight of each chemical element is the Time: 1 or 2 45 min class periods amount of grams per mole and this (processing own coconuts will require number is in the upper right hand corner and additional class period to prep for each element on the periodic table. coconut milk) For example Carbon is 12, Hydrogen 1, and Oxygen 16. The molecular weight of a molecule is equal to the sum of the Overview atomic weights. Students can then calculate the molecular weight of This curriculum is adaptable over a wide sucrose (C12H22O11), a disaccharide range of math and chemistry skill levels. composed of glucose (C6H12O6) and Examples of learning objectives include fructose (C6H12O6), which are mono- conversions from the English systems of saccharides. Most importantly engage measurement to the metric system of the students and have fun! measurement, utilizing multiplication, division, fractions, and decimals. Students can also use multiplication and division to determine serving size for HCPS III Mathematics each student and yield per coconut of Benchmarks Addressed: coconut sorbet. If a chemistry lesson is desired, students can calculate the MA.7.3.1 Multiplication and division density of an egg and perform the egg MA.7.1.1 Solve problems using test to approximate the percentage of fractions, decimals, and percents sugar in a given solution. -

Cedar Crest Ice Cream Products That Meet the USDA Smart Snacks in School Nutrition Standards
ORANGE YOU EXCITED ABOUT THIS NEW FLAVOR? Orange Vanilla Cedar Crest Ice Cream has developed a new sherbet and low fat ice cream flavor that meets the USDA’s Smart Snacks in School Nutrition Standards - Orange Vanilla. This new product is a flavor that school aged children will enjoy. Our flavor is a creamy sherbet and low fat ice cream product with an exceptional flavor profile. Please review the nutritional chart on the back side of this sheet and also the other Cedar Crest products that meet the USDA’s Smart Snacks in School Nutrition Standards. Cedar Crest Ice Cream 7269 Hwy 60 • P.O. Box 260 • Cedarburg, WI 53012 1-800-877-8341 • 262-377-7252 • Fax 262-377-5554 • www.cedarcresticecream.com Cedar Crest Ice Cream Products That Meet the USDA Smart Snacks in School Nutrition Standards Serving Vitamin Vitamin Product Item Calories Total Fat Sat Fat Trans Fat Cholesterol Sodium Carbohydrates Dietary Fiber Sugars Protein Calcium Iron Brand Serving Size Per Calories A C Description Number From Fat G (% Daily) G (% Daily) G (% Daily) Mg (% Daily) Mg (% Daily) G (% Daily) G (% Daily) G G % % Container % % Cedar Crest® 3 oz. Cups - Dairy Dessert Vanilla 3 fl.oz. (No Fat No Sugar 2120 Cedar Crest® 1 50 5 0 0 0 0 65 (3%) 13 (4%) 3 (12%) 4 2 0 0 6% 0 (49g) Added) Ingredients: Nonfat Milk, Polydextrose, Maltodextrin, Cream, Whey, Sorbitol, Guar Gum, Mono & Diglycerides, Xanthan Gum, Polysorbate 80, Carrageenan, Artificial Flavor, Caramel Color, Sucralose. Contains Milk. Chocolate Twist 3 fl.oz. (No Fat No Sugar 2121 Cedar Crest® 1 60 5 0 0 0 0 70 (3%) 15 (5%) 3 (12%) 3 2 0 0 6% 2% (52g) Added) Ingredients: Nonfat Milk, Polydextrose, Maltodextrin, Sorbitol, Guar Gum, Mono & Diglycerides, Xanthan Gum, Polysorbate 80, Carrageenan, Maltitol Syrup, Water, Glycerin, Cocoa, Cocoa (Processed with Alkali), Modified Food Starch, Salt, Citric Acid, Natural Flavors, Whey, Artificial Flavor, Caramel Color, Cream, Sucralose. -

Authentic Italiano Gelato | Sorbetto | PLANT-BASED | Desserts a Picture Is Just a Picture
AUTHENTIC ITALIANO GELATO | SORBETTO | PLANT-BASED | DESSERTS A picture is just a picture. But with passion, it is an art. Add our company passion and your work will become your vocation, your house will become home, your thoughts will become dreams, and your dreams will turn into reality. If you want anything strongly enough, you can find the willpower to achieve it. Passion changes you. In the end, gelato will be just gelato, but with passion, it is G.S. GELATO! Started with a facility less our story 1996 than 1,000 square feet Began national 1998 foodservice distribution Expanded production 2008 capabilities with a new 33,000 square foot facility Introduced Gelato & 2009 Sorbet product lines into the retail market Today we are the leaders 2010 timeline of achievements in foodservice and retail private label ALL BECAUSE TWO PEOPLE FELL IN LOVE Facility expansion included 2014 partial-system automation & G.S. Gelato is the story of love, passion, increased production capacity perseverance, and that “when you believe, all things are possible”. For three years in a row, G.S. Gelato With just their love for each other and a drive to 2015-17 was on the Inc. 5000 Fastest Growing achieve the American dream, Guido and Simona Private Companies list overcame all obstacles to become the first company to bring Italian gelato equipment to the United States. Introduced plant-based frozen dessert 2018 options that are Certified Vegan Today, as the leading gelato manufacturer for foodservice and retail private label, we welcome you to come along with us on our passionate journey in creating the world’s most exquisite Doubled production capacity and dessert creations.