Solvent-Free Aldol Condensation Reactions: Synthesis of Chalcone Derivatives Supplementary Material
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Aldrich FT-IR Collection Edition I Library
Aldrich FT-IR Collection Edition I Library Library Listing – 10,505 spectra This library is the original FT-IR spectral collection from Aldrich. It includes a wide variety of pure chemical compounds found in the Aldrich Handbook of Fine Chemicals. The Aldrich Collection of FT-IR Spectra Edition I library contains spectra of 10,505 pure compounds and is a subset of the Aldrich Collection of FT-IR Spectra Edition II library. All spectra were acquired by Sigma-Aldrich Co. and were processed by Thermo Fisher Scientific. Eight smaller Aldrich Material Specific Sub-Libraries are also available. Aldrich FT-IR Collection Edition I Index Compound Name Index Compound Name 3515 ((1R)-(ENDO,ANTI))-(+)-3- 928 (+)-LIMONENE OXIDE, 97%, BROMOCAMPHOR-8- SULFONIC MIXTURE OF CIS AND TRANS ACID, AMMONIUM SALT 209 (+)-LONGIFOLENE, 98+% 1708 ((1R)-ENDO)-(+)-3- 2283 (+)-MURAMIC ACID HYDRATE, BROMOCAMPHOR, 98% 98% 3516 ((1S)-(ENDO,ANTI))-(-)-3- 2966 (+)-N,N'- BROMOCAMPHOR-8- SULFONIC DIALLYLTARTARDIAMIDE, 99+% ACID, AMMONIUM SALT 2976 (+)-N-ACETYLMURAMIC ACID, 644 ((1S)-ENDO)-(-)-BORNEOL, 99% 97% 9587 (+)-11ALPHA-HYDROXY-17ALPHA- 965 (+)-NOE-LACTOL DIMER, 99+% METHYLTESTOSTERONE 5127 (+)-P-BROMOTETRAMISOLE 9590 (+)-11ALPHA- OXALATE, 99% HYDROXYPROGESTERONE, 95% 661 (+)-P-MENTH-1-EN-9-OL, 97%, 9588 (+)-17-METHYLTESTOSTERONE, MIXTURE OF ISOMERS 99% 730 (+)-PERSEITOL 8681 (+)-2'-DEOXYURIDINE, 99+% 7913 (+)-PILOCARPINE 7591 (+)-2,3-O-ISOPROPYLIDENE-2,3- HYDROCHLORIDE, 99% DIHYDROXY- 1,4- 5844 (+)-RUTIN HYDRATE, 95% BIS(DIPHENYLPHOSPHINO)BUT 9571 (+)-STIGMASTANOL -

(Nitroaldol) Reaction
MICROREVIEW DOI: 10.1002/ejoc.201101840 Biocatalytic Approaches to the Henry (Nitroaldol) Reaction Sinéad E. Milner,[a] Thomas S. Moody,[b] and Anita R. Maguire*[c] Keywords: Enzyme catalysis / Biocatalysis / C–C coupling / Nitroaldol reaction / Nitro alcohols Enantiopure β-nitro alcohols are key chiral building blocks approaches to the Henry (nitroaldol) reaction. The first for the synthesis of bioactive pharmaceutical ingredients. method is a direct enzyme-catalysed carbon–carbon bond The preparation of these target compounds in optically pure formation resulting in either an enantio-enriched or enantio- form has been the focus of much research and there has been pure β-nitro alcohol. The second approach describes the an emergence of biocatalytic protocols in the past decade. Henry reaction without stereocontrol followed by a biocata- For the first time, these biotransformations are the focus of lytic resolution to yield the enantiopure β-nitro alcohol. this review. Herein, we describe two principal biocatalytic Introduction The construction of carbon–carbon bonds is an essential element of synthetic organic chemistry. Among the various C–C bond forming reactions, the nitroaldol or Henry reac- tion[1] is one of the classical named reactions in organic synthesis. Essentially, this reaction describes the coupling of a nucleophilic nitro alkane with an electrophilic aldehyde or ketone to produce a synthetically useful β-nitro alcohol (Scheme 1).[2–5] Moreover, the Henry reaction facilitates the joining of two molecular fragments, under mild reaction conditions with the potential formation of two new ste- reogenic centres and a new C–C bond. The resulting β-nitro alcohols can undergo a variety of useful chemical transfor- mations which lead to synthetically useful structural motifs, e.g. -

Organocatalyzed Synthesis of Epoxides from Chalcones Utilizing Amino Acids
Western Michigan University ScholarWorks at WMU Master's Theses Graduate College 4-2018 Organocatalyzed Synthesis of Epoxides from Chalcones Utilizing Amino Acids Sabrina N. Kegeler Follow this and additional works at: https://scholarworks.wmich.edu/masters_theses Part of the Amino Acids, Peptides, and Proteins Commons, Medicinal-Pharmaceutical Chemistry Commons, and the Organic Chemistry Commons Recommended Citation Kegeler, Sabrina N., "Organocatalyzed Synthesis of Epoxides from Chalcones Utilizing Amino Acids" (2018). Master's Theses. 3418. https://scholarworks.wmich.edu/masters_theses/3418 This Masters Thesis-Open Access is brought to you for free and open access by the Graduate College at ScholarWorks at WMU. It has been accepted for inclusion in Master's Theses by an authorized administrator of ScholarWorks at WMU. For more information, please contact [email protected]. ORGANOCATALYZED SYNTHESIS OF EPOXIDES FROM CHALCONES UTILIZING AMINO ACIDS by Sabrina N. Kegeler A thesis submitted to the Graduate College in partial fulfillment of the requirements for the degree of Master of Science Chemistry Western Michigan University April 2018 Thesis Committee: James Kiddle, Ph.D., Chair Elke Schoffers, Ph.D. Gellert Mezei, Ph.D. Copyright by Sabrina N. Kegeler 2018 ORGANOCATALYZED SYNTHESIS OF EPOXIDES FROM CHALCONES UTILIZING AMINO ACIDS Sabrina N. Kegeler, M.S. Western Michigan University, 2018 The epoxide functional group is important throughout the chemical and pharmaceutical industries, as well as in nature. In the chemical industry, epoxides are present in resins and fragrances. In the pharmaceutical industry, epoxide-containing compounds are used as intermediates in the manufacturing of drugs. In nature, many natural products contain epoxide groups and are used for medicinal purposes, and for models to create synthetic molecules. -

Novel Methods for the Synthesis of Small Ring Systems
Novel Methods for the Synthesis of Small Ring Systems David Stephen Pugh Thesis submitted in partial fulfilment of the requirements for the degree of Doctor of Philosophy University of York Department of Chemistry September 2011 Abstract Chapter 1 briefly reviews telescoped reactions, in particular tandem oxidation processes, detailing the background to the development of the methodology presented here, and sets out the aims for the Thesis. Chapter 2 reviews cyclopropanation methods and examines the preparation and use of triisopropylsulfoxonium tetrafluoroborate II for the preparation of gem- dimethylcyclopropanes for a range of α,β-unsaturated compounds including ketones, esters, amides, nitriles and nitro-compounds (Scheme I). Chapter 2 also examines potential applications for the methodology, outlining a route towards the synthesis of chrysanthemic acid as well as attempts towards rearrangement methodology. Scheme I: Chapter 3 demonstrates the preparation of 3-substituted oxindoles from substituted anilides in a copper-mediated cyclisation-decarboxylation sequence (Scheme II). Scheme II: This sequence is demonstrated for a range of alkyl, aryl and heteroaryl substituents in the 3-position, with methyl, benzyl and PMB protection on the nitrogen atom. Further development of the copper-cyclisation methodology leading to a catalytic system is presented, along with initial work towards a decarboxylative allylation of oxindoles. i Contents Abstract i Contents ii List of tables and figures v Acknowledgements vi Declaration vii Chapter 1: -

Reactions of Benzene & Its Derivatives
Organic Lecture Series ReactionsReactions ofof BenzeneBenzene && ItsIts DerivativesDerivatives Chapter 22 1 Organic Lecture Series Reactions of Benzene The most characteristic reaction of aromatic compounds is substitution at a ring carbon: Halogenation: FeCl3 H + Cl2 Cl + HCl Chlorobenzene Nitration: H2 SO4 HNO+ HNO3 2 + H2 O Nitrobenzene 2 Organic Lecture Series Reactions of Benzene Sulfonation: H 2 SO4 HSO+ SO3 3 H Benzenesulfonic acid Alkylation: AlX3 H + RX R + HX An alkylbenzene Acylation: O O AlX H + RCX 3 CR + HX An acylbenzene 3 Organic Lecture Series Carbon-Carbon Bond Formations: R RCl AlCl3 Arenes Alkylbenzenes 4 Organic Lecture Series Electrophilic Aromatic Substitution • Electrophilic aromatic substitution: a reaction in which a hydrogen atom of an aromatic ring is replaced by an electrophile H E + + + E + H • In this section: – several common types of electrophiles – how each is generated – the mechanism by which each replaces hydrogen 5 Organic Lecture Series EAS: General Mechanism • A general mechanism slow, rate + determining H Step 1: H + E+ E El e ctro - Resonance-stabilized phile cation intermediate + H fast Step 2: E + H+ E • Key question: What is the electrophile and how is it generated? 6 Organic Lecture Series + + 7 Organic Lecture Series Chlorination Step 1: formation of a chloronium ion Cl Cl + + - - Cl Cl+ Fe Cl Cl Cl Fe Cl Cl Fe Cl4 Cl Cl Chlorine Ferric chloride A molecular complex An ion pair (a Lewis (a Lewis with a positive charge containing a base) acid) on ch lorine ch loronium ion Step 2: attack of -

Acetophenone Sigma Aldrich.Pdf
SIGMA-ALDRICH sigma-aldrich.com Material Safety Data Sheet Version 4.0 Revision Date 02/27/2010 Print Date 07/08/2011 1. PRODUCT AND COMPANY IDENTIFICATION Product name : Acetophenone Product Number : A10701 Brand : Sigma-Aldrich Company : Sigma-Aldrich 3050 Spruce Street SAINT LOUIS MO 63103 USA Telephone : +1 800-325-5832 Fax : +1 800-325-5052 Emergency Phone # : (314) 776-6555 2. HAZARDS IDENTIFICATION Emergency Overview OSHA Hazards Combustible Liquid, Harmful by ingestion., Irritant GHS Label elements, including precautionary statements Pictogram Signal word Danger Hazard statement(s) H227 Combustible liquid H302 Harmful if swallowed. H318 Causes serious eye damage. Precautionary statement(s) P210 Keep away from heat/sparks/open flames/hot surfaces. - No smoking. P264 Wash skin thoroughly after handling. P270 Do not eat, drink or smoke when using this product. P280 Wear protective gloves/protective clothing/eye protection/face protection. P301 + P312 IF SWALLOWED: Call a POISON CENTER or doctor/physician if you feel unwell. P305 + P351 + P338 IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. P310 Immediately call a POISON CENTER or doctor/physician. P330 Rinse mouth. P370 + P378 In case of fire: Use dry sand, dry chemical or alcohol-resistant foam for extinction. P403 + P235 Store in a well-ventilated place. Keep cool. P501 Dispose of contents/container to an approved waste disposal plant. HMIS Classification Health hazard: 2 Flammability: 2 Physical hazards: 0 NFPA Rating Health hazard: 2 Fire: 2 Reactivity Hazard: 0 Potential Health Effects Sigma-Aldrich - A10701 Page 1 of 6 Inhalation May be harmful if inhaled. -

Green Chemistry – Aspects for the Knoevenagel Reaction
2 Green Chemistry – Aspects for the Knoevenagel Reaction Ricardo Menegatti Universidade Federal de Goiás Brazil 1. Introduction Knoevenagel condensation is a classic C-C bond formation reaction in organic chemistry (Laue & Plagens, 2005). These condensations occur between aldehydes or ketones and active methylene compounds with ammonia or another amine as a catalyst in organic solvents (Knoevenagel, 1894). The Knoevenagel reaction is considered to be a modification of the aldol reaction; the main difference between these approaches is the higher acidity of the active methylene hydrogen when compared to an -carbonyl hydrogen (Smith & March, 2001). Figure 1 illustrates the condensation of a ketone (1) with a malonate compound (2) to form the Knoevenagel condensation product (3), which is then used to form the ,-unsaturated carboxylic compounds (3) and (4) (Laue & Plagens, 2005). R R R O O O O H O O O + base hydrolysis H O O O O R R (1) (2) (3) (4) Fig. 1. An example of the Knoevenagel reaction. Subsequent to the first description of the Knoevenagel reaction, changes were introduced using pyridine as the solvent and piperidine as the catalyst, which was named the Doebner Modification (Doebner, 1900). The Henry reaction is another variation of the Knoevenagel condensation that utilises compounds with an -nitro active methylene (Henry, 1895). The general mechanism for the Knoevenagel reaction, which involves deprotonation of the malonate derivative (6) by piperidine (5) and attack by the formed carbanion (8) on the carbonyl subunit (9) as an aldol reaction that forms the product (10) of the addition step is illustrated in Fig. -

ACETOPHENONE Method No. PV2003 Target Concentration
ACETOPHENONE Method no. PV2003 Target concentration: 100 ppm (491 mg/m3) Procedure: Samples are collected by drawing a known volume of air through a Tenax GC tube. Samples are desorbed with a solvent mixture of (5:95) Isopropanol:Carbon Disulfide (IPA):(CS2) and analyzed by gas chromatography with a flame ionization detector. Recommended air volume 120 minutes at 0.1 L/min (12 L) and sampling rate: Status of Method: Partially Validated. This method has been only partially evaluated and is presented for information and trial use. July 1982 Wayne Potter Service Branch 1 OSHA Salt Lake Technical Center Salt Lake City UT-84115 1 of 8 1 General Discussion 1.1 Background 1.1.1 History of procedure Recently, the OSHA Analytical Laboratory received a set of field samples that required analysis for acetophenone. The air samples had been collected on charcoal tubes and isopropanol impingers. Desorption studies were done on charcoal tubes using acetone, carbon disulfide, 5:95 isopropanol:carbon disulfide, and methylene chloride as desorbing solvents. The best results were obtained with 5:95 isopropanol:carbon disulfide, but the recovery was only 68%, which indicated that charcoal tubes should not be recommended for the collection of acetophenone air samples. An evaluation of isopropanol impingers was not performed because it was preferable to find an adsorbent procedure for sample collection. NIOSH method 291 for α -chloroacetophenone uses Tenax GC tubes. The Tenax GC tubes were tried for acetophenone, and appeared to be a suitable method of collection. 1.1.2 Toxic effects (This section is for information only and should not be taken as the basis of OSHA policy.) Acetophenone is a narcotic in high concentrations (Ref. -

Durham E-Theses
Durham E-Theses Functionalised Pyridyl- and Pyrimidyl- Boronic acids and derived new Biheteroaryls Smith, Amy Elizabeth How to cite: Smith, Amy Elizabeth (2005) Functionalised Pyridyl- and Pyrimidyl- Boronic acids and derived new Biheteroaryls, Durham theses, Durham University. Available at Durham E-Theses Online: http://etheses.dur.ac.uk/2751/ Use policy The full-text may be used and/or reproduced, and given to third parties in any format or medium, without prior permission or charge, for personal research or study, educational, or not-for-prot purposes provided that: • a full bibliographic reference is made to the original source • a link is made to the metadata record in Durham E-Theses • the full-text is not changed in any way The full-text must not be sold in any format or medium without the formal permission of the copyright holders. Please consult the full Durham E-Theses policy for further details. Academic Support Oce, Durham University, University Oce, Old Elvet, Durham DH1 3HP e-mail: [email protected] Tel: +44 0191 334 6107 http://etheses.dur.ac.uk 2 University of Durham A Thesis Entitled Functionalised Pyridyl- and Pyrimidyl- Boronic Acids and Derived New Biheteroaryls Submitted by Amy Elizabeth Smith, B.Sc. (Hons) (Ustinov College) Department of Chemistry A Candidate for the Degree of Doctor of Philosophy 2005 The copyright of this thesis rests wnh the author or the university to which к was submitted. No quotation from It, or Information derived from It may be published wHhout the prior written consent of the author or university, and any Information derived from ท should be acknowledged. -
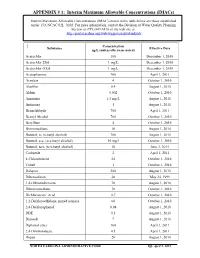
APPENDIX # 1: Interim Maximum Allowable Concentrations (Imacs)
APPENDIX # 1: Interim Maximum Allowable Concentrations (IMACs) Interim Maximum Allowable Concentrations (IMAC) shown in the table below are those established under 15A NCAC 02L .0202. For more information, contact the Division of Water Quality Planning Section at (919) 807-6416 or the web site at: http://portal.ncdenr.org/web/wq/ps/csu/gwstandards Concentration Substance Effective Date ug/L (unless otherwise noted) Acetochlor 100 December 1, 2010 Acetochlor ESA 1 mg/L December 1, 2010 Acetochlor OXA 1 mg/L December 1, 2010 Acetophenone 700 April 1, 2011 Acrolein 4 October 1, 2010 Alachlor 0.4 August 1, 2010 Aldrin 0.002 October 1, 2010 Ammonia 1.5 mg/L August 1, 2010 Antimony 1 August 1, 2010 Benzaldehyde 700 April 1, 2011 Benzyl Alcohol 700 October 1, 2010 Beryllium 4 October 1, 2010 Bromomethane 10 August 1, 2010 Butanol, n- (n-butyl alcohol) 700 August 1, 2010 Butanol, sec- (sec-butyl alcohol) 10 mg/l October 1, 2010 Butanol, tert- (tert-butyl alcohol) 10 June 1, 2011 Carbazole 2 April 1, 2011 4-Chlorotoluene 24 October 1, 2010 Cobalt 1 October 1, 2010 Dalapon 200 August 1, 2010 Dibenzofuran 28 May 24, 1999 1,4-Dibromobenzene 70 August 1, 2010 Dibromomethane 70 October 1, 2010 Dichloroacetic Acid 0.7 October 1, 2010 1,2-Dichloroethylene, mixed isomers 60 October 1, 2010 2,4-Dichlorophenol 0.98 August 1, 2010 DDE 0.1 August 1, 2010 Dinoseb 7 August 1, 2010 Diphenyl ether 100 April 1, 2011 2,4-Dinitrotoluene 0.1 April 1, 2011 Diquat 20 August 1, 2010 NORTH CAROLINA ADMINISTRATIVE CODE Eff. -

8.2 Shikimic Acid Pathway
CHAPTER 8 © Jones & Bartlett Learning, LLC © Jones & Bartlett Learning, LLC NOT FORAromatic SALE OR DISTRIBUTION and NOT FOR SALE OR DISTRIBUTION Phenolic Compounds © Jones & Bartlett Learning, LLC © Jones & Bartlett Learning, LLC NOT FOR SALE OR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION © Jones & Bartlett Learning, LLC © Jones & Bartlett Learning, LLC NOT FOR SALE OR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION © Jones & Bartlett Learning, LLC © Jones & Bartlett Learning, LLC NOT FOR SALE OR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION © Jones & Bartlett Learning, LLC © Jones & Bartlett Learning, LLC NOT FOR SALE OR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION © Jones & Bartlett Learning, LLC © Jones & Bartlett Learning, LLC NOT FOR SALE OR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION CHAPTER OUTLINE Overview Synthesis and Properties of Polyketides 8.1 8.5 Synthesis of Chalcones © Jones & Bartlett Learning, LLC © Jones & Bartlett Learning, LLC 8.2 Shikimic Acid Pathway Synthesis of Flavanones and Derivatives NOT FOR SALE ORPhenylalanine DISTRIBUTION and Tyrosine Synthesis NOT FOR SALESynthesis OR DISTRIBUTION and Properties of Flavones Tryptophan Synthesis Synthesis and Properties of Anthocyanidins Synthesis and Properties of Isofl avonoids Phenylpropanoid Pathway 8.3 Examples of Other Plant Polyketide Synthases Synthesis of Trans-Cinnamic Acid Synthesis and Activity of Coumarins Lignin Synthesis Polymerization© Jonesof Monolignols & Bartlett Learning, LLC © Jones & Bartlett Learning, LLC Genetic EngineeringNOT FOR of Lignin SALE OR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION Natural Products Derived from the 8.4 Phenylpropanoid Pathway Natural Products from Monolignols © Jones & Bartlett Learning, LLC © Jones & Bartlett Learning, LLC NOT FOR SALE OR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION © Jones & Bartlett Learning, LLC © Jones & Bartlett Learning, LLC NOT FOR SALE OR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION 119 © Jones & Bartlett Learning, LLC. -

200225128.Pdf
Members of the Examination Committee: Prof. Dr. ir. Nico Boon (Chairman) Department of Biotechnology, Faculty of Bioscience Engineering, Ghent University Prof. Dr. Adrian Dobbs Department of Pharmaceutical, Chemical and Environmental Sciences, Faculty of Engineering and Science, University of Greenwich Dr. Steven De Jonghe Laboratory of Virology and Chemotherapy, Rega Institute for Medical Research, KU Leuven Prof. Dr. Serge Van Calenbergh Laboratory of Medicinal Chemistry, Faculty of Pharmaceutical Sciences, Ghent University Prof. Dr. ir. Matthias D’hooghe Department of Green Chemistry and Technology, Faculty of Bioscience Engineering, Ghent University Prof. Dr. ir. Marjan De Mey Department of Biotechnology, Faculty of Bioscience Engineering, Ghent University This work was supported by the Agency for Innovation by Science and Technology, Flanders (IWT- SBO project 100014) and the Special Research Fund (BOF project BOF14/DC1/032) Promotor: Prof. Dr. ir. Christian Stevens Department of Green Chemistry and Technology Faculty of Bioscience Engineering Ghent University Dean: Prof. Dr. ir. Marc Van Meirvenne Rector: Prof. Dr. ir. Rik Van de Walle Faculty of Bioscience Engineering 2019 ir. Laurens De Coen Synthesis and Preliminary Biological Evaluation of Furo- and Oxazolopyrimidine Analogues Thesis submitted in fulfilment of the requirements for the degree of Doctor (PhD) in Applied Biological Sciences: Chemistry and Bioprocess Technology Dutch translation of the title: Synthese en preliminaire biologische evaluatie van furo- en oxazolopyrimidines Please cite as: De Coen, L.; ‘Synthesis and Preliminary Biological Evaluation of Furo- and Oxazolopyrimidine Analogues’, PhD Dissertation, Ghent University, 2019 Cover illustration: Exploring Chemical Space (Laurens De Coen) ISBN number: 9789463572064 The author and the promotor give authorization to consult and copy parts of this work for personal use only.