The Opossum Genome: Insights and Opportunities from an Alternative Mammal
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Evolution of the Patellar Sesamoid Bone in Mammals
A peer-reviewed version of this preprint was published in PeerJ on 21 March 2017. View the peer-reviewed version (peerj.com/articles/3103), which is the preferred citable publication unless you specifically need to cite this preprint. Samuels ME, Regnault S, Hutchinson JR. 2017. Evolution of the patellar sesamoid bone in mammals. PeerJ 5:e3103 https://doi.org/10.7717/peerj.3103 Evolution of the patellar sesamoid bone in mammals Mark E Samuels 1, 2 , Sophie Regnault 3 , John R Hutchinson Corresp. 3 1 Department of Medicine, University of Montreal, Montreal, Quebec, Canada 2 Centre de Recherche du CHU Ste-Justine, Montreal, Quebec, Canada 3 Structure & Motion Laboratory, Department of Comparative Biomedical Sciences, The Royal Veterinary College, Hatfield, Hertfordshire, United Kingdom Corresponding Author: John R Hutchinson Email address: [email protected] The patella is a sesamoid bone located in the major extensor tendon of the knee joint, in the hindlimb of many tetrapods. Although numerous aspects of knee morphology are ancient and conserved among most tetrapods, the evolutionary occurrence of an ossified patella is highly variable. Among extant (crown clade) groups it is found in most birds, most lizards, the monotreme mammals and almost all placental mammals, but it is absent in most marsupial mammals as well as many reptiles. Here we integrate data from the literature and first-hand studies of fossil and recent skeletal remains to reconstruct the evolution of the mammalian patella. We infer that bony patellae most likely evolved between four to six times in crown group Mammalia: in monotremes, in the extinct multituberculates, in one or more stem-mammal genera outside of therian or eutherian mammals, and up to three times in therian mammals. -

Monodelphis Domestica in the Opossum Λ Conservation Of
Marsupial Light Chains: Complexity and Conservation of λ in the Opossum Monodelphis domestica This information is current as Julie E. Lucero, George H. Rosenberg and Robert D. Miller of September 29, 2021. J Immunol 1998; 161:6724-6732; ; http://www.jimmunol.org/content/161/12/6724 Downloaded from References This article cites 35 articles, 10 of which you can access for free at: http://www.jimmunol.org/content/161/12/6724.full#ref-list-1 Why The JI? Submit online. http://www.jimmunol.org/ • Rapid Reviews! 30 days* from submission to initial decision • No Triage! Every submission reviewed by practicing scientists • Fast Publication! 4 weeks from acceptance to publication *average by guest on September 29, 2021 Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 1998 by The American Association of Immunologists All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. Marsupial Light Chains: Complexity and Conservation of l in the Opossum Monodelphis domestica1,2 Julie E. Lucero, George H. Rosenberg, and Robert D. Miller3 The Igl chains in the South American opossum, Monodelphis domestica, were analyzed at the expressed cDNA and genomic organization level, the first described for a nonplacental mammal. -

Constraints on the Timescale of Animal Evolutionary History
Palaeontologia Electronica palaeo-electronica.org Constraints on the timescale of animal evolutionary history Michael J. Benton, Philip C.J. Donoghue, Robert J. Asher, Matt Friedman, Thomas J. Near, and Jakob Vinther ABSTRACT Dating the tree of life is a core endeavor in evolutionary biology. Rates of evolution are fundamental to nearly every evolutionary model and process. Rates need dates. There is much debate on the most appropriate and reasonable ways in which to date the tree of life, and recent work has highlighted some confusions and complexities that can be avoided. Whether phylogenetic trees are dated after they have been estab- lished, or as part of the process of tree finding, practitioners need to know which cali- brations to use. We emphasize the importance of identifying crown (not stem) fossils, levels of confidence in their attribution to the crown, current chronostratigraphic preci- sion, the primacy of the host geological formation and asymmetric confidence intervals. Here we present calibrations for 88 key nodes across the phylogeny of animals, rang- ing from the root of Metazoa to the last common ancestor of Homo sapiens. Close attention to detail is constantly required: for example, the classic bird-mammal date (base of crown Amniota) has often been given as 310-315 Ma; the 2014 international time scale indicates a minimum age of 318 Ma. Michael J. Benton. School of Earth Sciences, University of Bristol, Bristol, BS8 1RJ, U.K. [email protected] Philip C.J. Donoghue. School of Earth Sciences, University of Bristol, Bristol, BS8 1RJ, U.K. [email protected] Robert J. -

0 Introduction
Cambridge University Press 978-0-521-78117-6 - Evolution of Tertiary Mammals of North America, Volume 2: Small Mammals, Xenarthrans, and Marine Mammals Christine M. Janis, Gregg F. Gunnell and Mark D. Uhen Excerpt More information 0 Introduction christine m. janis,1 gregg f. gunnell2 and mark d. uhen3 1Brown University, Providence, RI, USA 2Museum of Paleontology, University of Michigan, Ann Arbor, MI, USA 3Smithsonian Institution, Washington, DC, USA AIMS OF VOLUME 2 the chapter are presented according to a standardized format, and the institutional abbreviations have also been standardized and are This enterprise was originally conceived of as a single volume. How- listed in an appendix (Appendix III). ever, after a span of 10 years from its original conception, the current senior editor (Christine Janis), and the then junior editors (Kathleen Scott and Louis Jacobs) realized that it would be more realistic THE STANDARDIZED LAYOUT OF EACH CHAPTER to proceed with chapters then in hand, which could more or less be assembled into the conceptually useful, if taxonomically para- The chapters are laid out in a similar fashion to those in Volume 1. phyletic, rubric of “Terrestrial Carnivores and Ungulates” (Janis, The contributors were requested to adhere to a common layout for Scott, and Jacobs, 1998). This in part reflected the chapters that had each chapter, in order to provide uniform information throughout been assembled to date, although it should be noted that some of the book. The “Introduction” for each chapter introduces the group. the chapters in this current volume, most notably those by Darryl The “Defining features” section lays out the basic cranial, dental, Domning on sirenians and desmostylians, were among the first ones and postcranial features of the taxon. -

OPOSSUM Didelphis Virginiana
OPOSSUM Didelphis virginiana The Virginia opossum, Didelphis virginiana, is the only marsupial (pouched animal) native to North America. The opossum is not a native species to Vermont, but a population has become established here. The opossum is mostly active at night, being what is referred to as ‘nocturnal.’ They are very good climbers and capable swimmers. These two skills help the opossum avoid predators. It is well known for faking death (also called ‘playing possum’) as another means of outwitting its enemies. The opossum adapts to a wide variety of habitats which has led to its widespread distribution throughout the United States. Vermont Wildlife Fact Sheet Physical Description Opossums breed every other areas near water sources. year, having one litter every They have become very The fur of the Virginia two years. Opossums reach common in urban, suburban, opossum is grayish white in the age of sexual maturity at 6 and farming areas. The color and covers the whole to 7 months. opossum is a wanderer and body except the ears and tail. does not stick to a specific They are about the size of a Food Items territory. The opossum uses large house cat, weighing abandoned burrows, tree between 9 and 13 pounds and The opossum is an cavities, hollow logs, attics, having a body length of 24 to insectivore and an omnivore. garages, or building 40 inches. The opossum has a This means they have a foundations. prehensile tail, one which is varied diet of insects, worms, adapted for grasping and fruits, nuts, and carrion (dead hanging. animals). -

Dominance Relationships in Captive Male Bare-Tailed Woolly Opossum (Caluromys Phiiander, Marsupialia: Didelphidae)
DOMINANCE RELATIONSHIPS IN CAPTIVE MALE BARE-TAILED WOOLLY OPOSSUM (CALUROMYS PHIIANDER, MARSUPIALIA: DIDELPHIDAE) M.-L. GUILLEMIN*, M. ATRAMENTOWICZ* & P. CHARLES-DOMINIQUE* RÉSUMÉ Au cours de ce travail nous avons voulu tester en captivité l'importance du poids corporel dans l'établissement de relations de dominance chez les mâles Caluromysphilander, chez qui des compétitions inter-mâles ont été étudiées. Les comportements et l'évolution de différents paramètres physiologiques ont été observés durant 18 expérimentations effectuées respectivement sur 6 groupes de deux mâles et sur 12 groupes de deux mâles et une femelle. Des relations de dominance-subordination se mettent en place même en l'absence de femelle, mais la compétition est plus forte dans les groupes comprenant une femelle. Dans ces conditions expérimentales, le rang social est basé principalement sur le poids et l'âge. Lorsque la relation de dominance est mise en place, le rang social des mâles est bien défini et il reste stable jusqu'à la fin de l'expérimentation. Ces relations de dominance stables pourraient profiter aux dominants et aux dominés en minimisant les risques de blessures sérieuses. Les mâles montrent des signes typiques caractérisant un stress social : une baisse du poids et de l'hématocrite, les dominés étant plus stressés que les dominants. Chez les mâles dominants, la baisse de l'hématocrite est plus faible que chez les dominés, et la concentration de testostérone dans le sang diminue plus que chez les dominés. Au niveau comportemental, les dominants effectuent la plupart des interactions agonistiques << offensives » et plus d'investigations olfactives de leur environnement (flairage-léchage) que les dominés. -

The Oldest Platypus and Its Bearing on Divergence Timing of the Platypus and Echidna Clades
The oldest platypus and its bearing on divergence timing of the platypus and echidna clades Timothy Rowe*†, Thomas H. Rich‡§, Patricia Vickers-Rich§, Mark Springer¶, and Michael O. Woodburneʈ *Jackson School of Geosciences, University of Texas, C1100, Austin, TX 78712; ‡Museum Victoria, PO Box 666, Melbourne, Victoria 3001, Australia; §School of Geosciences, PO Box 28E, Monash University, Victoria 3800, Australia; ¶Department of Biology, University of California, Riverside, CA 92521; and ʈDepartment of Geology, Museum of Northern Arizona, Flagstaff, AZ 86001 Edited by David B. Wake, University of California, Berkeley, CA, and approved October 31, 2007 (received for review July 7, 2007) Monotremes have left a poor fossil record, and paleontology has broadly affect our understanding of early mammalian history, been virtually mute during two decades of discussion about with special implications for molecular clock estimates of basal molecular clock estimates of the timing of divergence between the divergence times. platypus and echidna clades. We describe evidence from high- Monotremata today comprises five species that form two resolution x-ray computed tomography indicating that Teinolo- distinct clades (16). The echidna clade includes one short-beaked phos, an Early Cretaceous fossil from Australia’s Flat Rocks locality species (Tachyglossus aculeatus; Australia and surrounding is- (121–112.5 Ma), lies within the crown clade Monotremata, as a lands) and three long-beaked species (Zaglossus bruijni, Z. basal platypus. Strict molecular clock estimates of the divergence bartoni, and Z. attenboroughi, all from New Guinea). The between platypus and echidnas range from 17 to 80 Ma, but platypus clade includes only Ornithorhynchus anatinus (Austra- Teinolophos suggests that the two monotreme clades were al- lia, Tasmania). -

Marsupial in Maine: Opossum
Maine Bureau of Parks and Lands www.parksandlands.com Marsupial in Maine: Opossum (Originally published 7/1/2020) If Australia and kangaroos come to mind when you think of marsupials, you are correct. But Maine has one - the Virginia opossum (Didelphis virginianus). Marsupials are mammals that do not give birth to fully developed young. The young are instead born when they are extremely tiny and must then crawl to the mother’s pouch. There, they will suckle milk and continue to grow for many weeks. Baby opossums, called pups or pinkies at birth, are no bigger than a honeybee. Curled up on their side, they are no larger round than a dime and weigh in at approximately .13 grams. This is less than a dime, which weighs 2.268 grams (0.080 ounces)! After a week in their mother’s pouch, their birth weight will have increased by ten times. Opossum are skilled tree climbers. Photo by Kim Chandler. After two months, they are mouse-size and will begin exploring briefly outside the pouch. In another month, they will spend more time outside the pouch and may be carried on their mother’s back. They cling tightly to her fur with their hand-like feet and grasping tails. The night-active (nocturnal) opossum is not a fussy eater. It prefers to stay close to its den but may roam up to two miles nightly in search of food. While ambling along trails near a wetland or stream, it will look for insects, worms, frogs, plants, and seeds to eat. Roads, also visited during the nighttime, are sources of dead animals that the opossum will eat. -

Controlling Raccoon and Opossum Damage
G1688 Controlling Raccoon and Opossum Damage Stephen Vantassel, Extension Project Coordinator-Wildlife Damage Management Scott Hygnstrom, Extension Specialist-Wildlife Damage Management Dennis Ferraro, Extension Educator—Douglas-Sarpy County Sam Wilson, Furbearer Biologist—Nebraska Game & Parks Commission a shingled roof to establish a den in an attic. Raccoons usu- This NebGuide provides information about rac- ally need only a 4- to 6-inch diameter hole to enter. Smaller coons and opossums, the damage they cause, and ways openings are often enlarged. Raccoons are very strong and to prevent and control damage problems. have tremendous dexterity in their front paws. Despite their size, raccoons are exceptional climbers, able to scale trees, chimneys, and downspouts. Raccoons and opossums are nocturnal, medium-sized Raccoons are omnivores, enjoying a diet ranging from terrestrial mammals that exist throughout Nebraska. They eggs, carrion, food scraps, corn, crayfish, bird seed, worms, can damage lawns and gardens, scatter trash, invade buildings amphibians, pet food and more. While extremely harsh and injure livestock. weather will cause them to remain in their dens, raccoons do not hibernate. Raccoon Facts Opossum Facts Raccoons, with their black bandit Opossums (Didel mask and black- and phis virginiana) have brown-banded tail, white to gray coloration are well known to in their fur, grow to Nebraskans (Figure the size of housecats 1). The word “rac- and weigh around 10 coon” is derived from pounds (Figure 3). Their Native American pointed face and rat-like words “arakum” or tail lead many people “aracoun,” meaning to consider them to be Figure 3. Opossum. Figure 1. Raccoon on shingled roof. -

The Biodynamics of Arboreal Locomotion in the Gray Short
THE BIODYNAMICS OF ARBOREAL LOCOMOTION IN THE GRAY SHORT- TAILED OPOSSUM (MONODELPHIS DOMESTICA) A dissertation presented to the faculty of the College of Arts and Sciences of Ohio University In partial fulfillment of the requirements for the degree Doctor of Philosophy Andrew R. Lammers August 2004 This dissertation entitled THE BIODYNAMICS OF ARBOREAL LOCOMOTION IN THE GRAY SHORT- TAILED OPOSSUM (MONODELPHIS DOMESTICA) BY ANDREW R. LAMMERS has been approved for the Department of Biological Sciences and the College of Arts and Sciences by Audrone R. Biknevicius Associate Professor of Biomedical Sciences Leslie A. Flemming Dean, College of Arts and Sciences LAMMERS, ANDREW R. Ph.D. August 2004. Biological Sciences The biodynamics of arboreal locomotion in the gray short-tailed opossum (Monodelphis domestica). (147 pp.) Director of Dissertation: Audrone R. Biknevicius Most studies of animal locomotor biomechanics examine movement on a level, flat trackway. However, small animals must negotiate heterogenerous terrain that includes changes in orientation and diameter. Furthermore, animals which are specialized for arboreal locomotion may solve the biomechanical problems that are inherent in substrates that are sloped and/or narrow differently from animals which are considered terrestrial. Thus I studied the effects of substrate orientation and diameter on locomotor kinetics and kinematics in the gray short-tailed opossum (Monodelphis domestica). The genus Monodelphis is considered the most terrestrially adapted member of the family Didelphidae, but nevertheless these opossums are reasonably skilled at climbing. The first study (Chapter 2) examines the biomechanics of moving up a 30° incline and down a 30° decline. Substrate reaction forces (SRFs), limb kinematics, and required coefficient of friction were measured. -
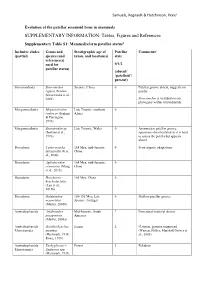
SUPPLEMENTARY INFORMATION: Tables, Figures and References
Samuels, Regnault & Hutchinson, PeerJ Evolution of the patellar sesamoid bone in mammals SUPPLEMENTARY INFORMATION: Tables, Figures and References Supplementary Table S1: Mammaliaform patellar status$ Inclusive clades Genus and Stratigraphic age of Patellar Comments# (partial) species (and taxon, and location(s) state reference(s) used for 0/1/2 patellar status) (absent/ ‘patelloid’/ present) Sinoconodonta Sinoconodon Jurassic, China 0 Patellar groove absent, suggests no rigneyi (Kielan- patella Jaworowska et al., 2004) Sinoconodon is included on our phylogeny within tritylodontids. Morganucodonta Megazostrodon Late Triassic, southern 0 rudnerae (Jenkins Africa & Parrington, 1976) Morganucodonta Eozostrodon sp. Late Triassic, Wales 0 Asymmetric patellar groove, (Jenkins et al., specimens disarticulated so it is hard 1976) to assess the patella but appears absent Docodonta Castorocauda 164 Mya, mid-Jurassic, 0 Semi-aquatic adaptations lutrasimilis (Ji et China al., 2006) Docodonta Agilodocodon 164 Mya, mid-Jurassic, 0 scansorius (Meng China et al., 2015) Docodonta Docofossor 160 Mya, China 0 brachydactylus (Luo et al., 2015b) Docodonta Haldanodon 150-155 Mya, Late 0 Shallow patellar groove exspectatus Jurassic, Portugal (Martin, 2005b) Australosphenida Asfaltomylos Mid-Jurassic, South ? Postcranial material absent patagonicus America (Martin, 2005a) Australosphenida Ornithorhynchus Extant 2 Platypus, genome sequenced Monotremata anatinus (Warren, Hillier, Marshall Graves et (Herzmark, 1938; al., 2008) Rowe, 1988) Australosphenida Tachyglossus -

Eutheria (Placental Mammals)
Eutheria (Placental Introductory article Mammals) Article Contents . Introduction J David Archibald, San Diego State University, San Diego, California, USA . Basic Design . Taxonomic and Ecological Diversity Eutheria includes one of three major clades of mammals, the extant members of which are . Fossil History and Distribution referred to as placentals. Phylogeny Introduction have supernumerary teeth (e.g. some whales, armadillos, Eutheria (or Placentalia) is the most taxonomically diverse etc.), in extant placentals the number of teeth is at most of three branches or clades of mammals, the other two three upper and lower incisors, one upper and lower being Metatheria (or Marsupialia) and Prototheria (or canine, four upper and lower premolars, and three upper Monotremata). When named by Gill in 1872, Eutheria and lower molars. Except for one fewer upper molar, a included both marsupials and placentals. It was Huxley in domestic dog retains this pattern. Compared to reptiles, 1880 that recognized Eutheria basically as used today to mammals have fewer skull bones through fusion and loss, include only placentals. McKenna and Bell in their although bones are variously emphasized in each of the Classification of Mammals, published in 1997, chose to three major mammalian taxa. use Placentalia rather than Eutheria to avoid the confusion Physiologically, mammals are all endotherms of varying of what taxa should be included in Eutheria. Others such as degrees of efficiency. They are also homeothermic with a Rougier have used Eutheria and Placentalia in the sense relatively high resting temperature. These characteristics used here. Placentalia includes all extant placentals and are also found in birds, but because of anatomical their most recent common ancestor.