Introduction to Nuclear Physics
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Glossary Physics (I-Introduction)
1 Glossary Physics (I-introduction) - Efficiency: The percent of the work put into a machine that is converted into useful work output; = work done / energy used [-]. = eta In machines: The work output of any machine cannot exceed the work input (<=100%); in an ideal machine, where no energy is transformed into heat: work(input) = work(output), =100%. Energy: The property of a system that enables it to do work. Conservation o. E.: Energy cannot be created or destroyed; it may be transformed from one form into another, but the total amount of energy never changes. Equilibrium: The state of an object when not acted upon by a net force or net torque; an object in equilibrium may be at rest or moving at uniform velocity - not accelerating. Mechanical E.: The state of an object or system of objects for which any impressed forces cancels to zero and no acceleration occurs. Dynamic E.: Object is moving without experiencing acceleration. Static E.: Object is at rest.F Force: The influence that can cause an object to be accelerated or retarded; is always in the direction of the net force, hence a vector quantity; the four elementary forces are: Electromagnetic F.: Is an attraction or repulsion G, gravit. const.6.672E-11[Nm2/kg2] between electric charges: d, distance [m] 2 2 2 2 F = 1/(40) (q1q2/d ) [(CC/m )(Nm /C )] = [N] m,M, mass [kg] Gravitational F.: Is a mutual attraction between all masses: q, charge [As] [C] 2 2 2 2 F = GmM/d [Nm /kg kg 1/m ] = [N] 0, dielectric constant Strong F.: (nuclear force) Acts within the nuclei of atoms: 8.854E-12 [C2/Nm2] [F/m] 2 2 2 2 2 F = 1/(40) (e /d ) [(CC/m )(Nm /C )] = [N] , 3.14 [-] Weak F.: Manifests itself in special reactions among elementary e, 1.60210 E-19 [As] [C] particles, such as the reaction that occur in radioactive decay. -

The Five Common Particles
The Five Common Particles The world around you consists of only three particles: protons, neutrons, and electrons. Protons and neutrons form the nuclei of atoms, and electrons glue everything together and create chemicals and materials. Along with the photon and the neutrino, these particles are essentially the only ones that exist in our solar system, because all the other subatomic particles have half-lives of typically 10-9 second or less, and vanish almost the instant they are created by nuclear reactions in the Sun, etc. Particles interact via the four fundamental forces of nature. Some basic properties of these forces are summarized below. (Other aspects of the fundamental forces are also discussed in the Summary of Particle Physics document on this web site.) Force Range Common Particles It Affects Conserved Quantity gravity infinite neutron, proton, electron, neutrino, photon mass-energy electromagnetic infinite proton, electron, photon charge -14 strong nuclear force ≈ 10 m neutron, proton baryon number -15 weak nuclear force ≈ 10 m neutron, proton, electron, neutrino lepton number Every particle in nature has specific values of all four of the conserved quantities associated with each force. The values for the five common particles are: Particle Rest Mass1 Charge2 Baryon # Lepton # proton 938.3 MeV/c2 +1 e +1 0 neutron 939.6 MeV/c2 0 +1 0 electron 0.511 MeV/c2 -1 e 0 +1 neutrino ≈ 1 eV/c2 0 0 +1 photon 0 eV/c2 0 0 0 1) MeV = mega-electron-volt = 106 eV. It is customary in particle physics to measure the mass of a particle in terms of how much energy it would represent if it were converted via E = mc2. -

Hetc-3Step Calculations of Proton Induced Nuclide Production Cross Sections at Incident Energies Between 20 Mev and 5 Gev
JAERI-Research 96-040 JAERI-Research—96-040 JP9609132 JP9609132 HETC-3STEP CALCULATIONS OF PROTON INDUCED NUCLIDE PRODUCTION CROSS SECTIONS AT INCIDENT ENERGIES BETWEEN 20 MEV AND 5 GEV August 1996 Hiroshi TAKADA, Nobuaki YOSHIZAWA* and Kenji ISHIBASHI* Japan Atomic Energy Research Institute 2 G 1 0 1 (T319-11 l This report is issued irregularly. Inquiries about availability of the reports should be addressed to Research Information Division, Department of Intellectual Resources, Japan Atomic Energy Research Institute, Tokai-mura, Naka-gun, Ibaraki-ken, 319-11, Japan. © Japan Atomic Energy Research Institute, 1996 JAERI-Research 96-040 HETC-3STEP Calculations of Proton Induced Nuclide Production Cross Sections at Incident Energies between 20 MeV and 5 GeV Hiroshi TAKADA, Nobuaki YOSHIZAWA* and Kenji ISHIBASHP* Department of Reactor Engineering Tokai Research Establishment Japan Atomic Energy Research Institute Tokai-mura, Naka-gun, Ibaraki-ken (Received July 1, 1996) For the OECD/NEA code intercomparison, nuclide production cross sections of l60, 27A1, nalFe, 59Co, natZr and 197Au for the proton incidence with energies of 20 MeV to 5 GeV are calculated with the HETC-3STEP code based on the intranuclear cascade evaporation model including the preequilibrium and high energy fission processes. In the code, the level density parameter derived by Ignatyuk, the atomic mass table of Audi and Wapstra and the mass formula derived by Tachibana et al. are newly employed in the evaporation calculation part. The calculated results are compared with the experimental ones. It is confirmed that HETC-3STEP reproduces the production of the nuclides having the mass number close to that of the target nucleus with an accuracy of a factor of two to three at incident proton energies above 100 MeV for natZr and 197Au. -

Probing Pulse Structure at the Spallation Neutron Source Via Polarimetry Measurements
University of Tennessee, Knoxville TRACE: Tennessee Research and Creative Exchange Masters Theses Graduate School 5-2017 Probing Pulse Structure at the Spallation Neutron Source via Polarimetry Measurements Connor Miller Gautam University of Tennessee, Knoxville, [email protected] Follow this and additional works at: https://trace.tennessee.edu/utk_gradthes Part of the Nuclear Commons Recommended Citation Gautam, Connor Miller, "Probing Pulse Structure at the Spallation Neutron Source via Polarimetry Measurements. " Master's Thesis, University of Tennessee, 2017. https://trace.tennessee.edu/utk_gradthes/4741 This Thesis is brought to you for free and open access by the Graduate School at TRACE: Tennessee Research and Creative Exchange. It has been accepted for inclusion in Masters Theses by an authorized administrator of TRACE: Tennessee Research and Creative Exchange. For more information, please contact [email protected]. To the Graduate Council: I am submitting herewith a thesis written by Connor Miller Gautam entitled "Probing Pulse Structure at the Spallation Neutron Source via Polarimetry Measurements." I have examined the final electronic copy of this thesis for form and content and recommend that it be accepted in partial fulfillment of the equirr ements for the degree of Master of Science, with a major in Physics. Geoffrey Greene, Major Professor We have read this thesis and recommend its acceptance: Marianne Breinig, Nadia Fomin Accepted for the Council: Dixie L. Thompson Vice Provost and Dean of the Graduate School (Original signatures are on file with official studentecor r ds.) Probing Pulse Structure at the Spallation Neutron Source via Polarimetry Measurements A Thesis Presented for the Master of Science Degree The University of Tennessee, Knoxville Connor Miller Gautam May 2017 c by Connor Miller Gautam, 2017 All Rights Reserved. -

B2.IV Nuclear and Particle Physics
B2.IV Nuclear and Particle Physics A.J. Barr February 13, 2014 ii Contents 1 Introduction 1 2 Nuclear 3 2.1 Structure of matter and energy scales . 3 2.2 Binding Energy . 4 2.2.1 Semi-empirical mass formula . 4 2.3 Decays and reactions . 8 2.3.1 Alpha Decays . 10 2.3.2 Beta decays . 13 2.4 Nuclear Scattering . 18 2.4.1 Cross sections . 18 2.4.2 Resonances and the Breit-Wigner formula . 19 2.4.3 Nuclear scattering and form factors . 22 2.5 Key points . 24 Appendices 25 2.A Natural units . 25 2.B Tools . 26 2.B.1 Decays and the Fermi Golden Rule . 26 2.B.2 Density of states . 26 2.B.3 Fermi G.R. example . 27 2.B.4 Lifetimes and decays . 27 2.B.5 The flux factor . 28 2.B.6 Luminosity . 28 2.C Shell Model § ............................. 29 2.D Gamma decays § ............................ 29 3 Hadrons 33 3.1 Introduction . 33 3.1.1 Pions . 33 3.1.2 Baryon number conservation . 34 3.1.3 Delta baryons . 35 3.2 Linear Accelerators . 36 iii CONTENTS CONTENTS 3.3 Symmetries . 36 3.3.1 Baryons . 37 3.3.2 Mesons . 37 3.3.3 Quark flow diagrams . 38 3.3.4 Strangeness . 39 3.3.5 Pseudoscalar octet . 40 3.3.6 Baryon octet . 40 3.4 Colour . 41 3.5 Heavier quarks . 43 3.6 Charmonium . 45 3.7 Hadron decays . 47 Appendices 48 3.A Isospin § ................................ 49 3.B Discovery of the Omega § ...................... -

Nuclear Models: Shell Model
LectureLecture 33 NuclearNuclear models:models: ShellShell modelmodel WS2012/13 : ‚Introduction to Nuclear and Particle Physics ‘, Part I 1 NuclearNuclear modelsmodels Nuclear models Models with strong interaction between Models of non-interacting the nucleons nucleons Liquid drop model Fermi gas model ααα-particle model Optical model Shell model … … Nucleons interact with the nearest Nucleons move freely inside the nucleus: neighbors and practically don‘t move: mean free path λ ~ R A nuclear radius mean free path λ << R A nuclear radius 2 III.III. ShellShell modelmodel 3 ShellShell modelmodel Magic numbers: Nuclides with certain proton and/or neutron numbers are found to be exceptionally stable. These so-called magic numbers are 2, 8, 20, 28, 50, 82, 126 — The doubly magic nuclei: — Nuclei with magic proton or neutron number have an unusually large number of stable or long lived nuclides . — A nucleus with a magic neutron (proton) number requires a lot of energy to separate a neutron (proton) from it. — A nucleus with one more neutron (proton) than a magic number is very easy to separate. — The first exitation level is very high : a lot of energy is needed to excite such nuclei — The doubly magic nuclei have a spherical form Nucleons are arranged into complete shells within the atomic nucleus 4 ExcitationExcitation energyenergy forfor magicm nuclei 5 NuclearNuclear potentialpotential The energy spectrum is defined by the nuclear potential solution of Schrödinger equation for a realistic potential The nuclear force is very short-ranged => the form of the potential follows the density distribution of the nucleons within the nucleus: for very light nuclei (A < 7), the nucleon distribution has Gaussian form (corresponding to a harmonic oscillator potential ) for heavier nuclei it can be parameterised by a Fermi distribution. -

An Octad for Darmstadtium and Excitement for Copernicium
SYNOPSIS An Octad for Darmstadtium and Excitement for Copernicium The discovery that copernicium can decay into a new isotope of darmstadtium and the observation of a previously unseen excited state of copernicium provide clues to the location of the “island of stability.” By Katherine Wright holy grail of nuclear physics is to understand the stability uncover its position. of the periodic table’s heaviest elements. The problem Ais, these elements only exist in the lab and are hard to The team made their discoveries while studying the decay of make. In an experiment at the GSI Helmholtz Center for Heavy isotopes of flerovium, which they created by hitting a plutonium Ion Research in Germany, researchers have now observed a target with calcium ions. In their experiments, flerovium-288 previously unseen isotope of the heavy element darmstadtium (Z = 114, N = 174) decayed first into copernicium-284 and measured the decay of an excited state of an isotope of (Z = 112, N = 172) and then into darmstadtium-280 (Z = 110, another heavy element, copernicium [1]. The results could N = 170), a previously unseen isotope. They also measured an provide “anchor points” for theories that predict the stability of excited state of copernicium-282, another isotope of these heavy elements, says Anton Såmark-Roth, of Lund copernicium. Copernicium-282 is interesting because it University in Sweden, who helped conduct the experiments. contains an even number of protons and neutrons, and researchers had not previously measured an excited state of a A nuclide’s stability depends on how many protons (Z) and superheavy even-even nucleus, Såmark-Roth says. -
Pair Energy of Proton and Neutron in Atomic Nuclei
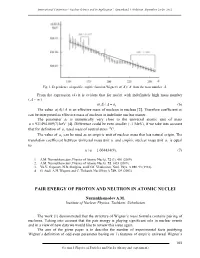
International Conference “Nuclear Science and its Application”, Samarkand, Uzbekistan, September 25-28, 2012 Fig. 1. Dependence of specific empiric function Wigner's a()/ A A from the mass number A. From the expression (4) it is evident that for nuclei with indefinitely high mass number ( A ~ ) a()/. A A a1 (6) The value a()/ A A is an effective mass of nucleon in nucleus [2]. Therefore coefficient a1 can be interpreted as effective mass of nucleon in indefinite nuclear matter. The parameter a1 is numerically very close to the universal atomic unit of mass u 931494.009(7) keV [4]. Difference could be even smaller (~1 MeV), if we take into account that for definition of u, used mass of neutral atom 12C. The value of a1 can be used as an empiric unit of nuclear mass that has natural origin. The translation coefficient between universal mass unit u and empiric nuclear mass unit a1 is equal to: u/ a1 1.004434(9). (7) 1. A.M. Nurmukhamedov, Physics of Atomic Nuclei, 72 (3), 401 (2009). 2. A.M. Nurmukhamedov, Physics of Atomic Nuclei, 72, 1435 (2009). 3. Yu.V. Gaponov, N.B. Shulgina, and D.M. Vladimirov, Nucl. Phys. A 391, 93 (1982). 4. G. Audi, A.H. Wapstra and C. Thibault, Nucl.Phys A 729, 129 (2003). PAIR ENERGY OF PROTON AND NEUTRON IN ATOMIC NUCLEI Nurmukhamedov A.M. Institute of Nuclear Physics, Tashkent, Uzbekistan The work [1] demonstrated that the structure of Wigner’s mass formula contains pairing of nucleons. Taking into account that the pair energy is playing significant role in nuclear events and in a view of new data we would like to review this issue again. -

Evolution of Intrinsic Nuclear Structure in Medium Mass Even-Even Xenon Isotopes from a Microscopic Perspective*
Chinese Physics C Vol. 44, No. 7 (2020) 074108 Evolution of intrinsic nuclear structure in medium mass even-even Xenon isotopes from a microscopic perspective* Surbhi Gupta1 Ridham Bakshi1 Suram Singh2 Arun Bharti1;1) G. H. Bhat3 J. A. Sheikh4 1Department of Physics, University of Jammu, Jammu- 180006, India 2Department of Physics and Astronomical Sciences, Central University of Jammu, Samba- 181143, India 3Department of Physics, SP College, Cluster University Srinagar- 190001, India 4Cluster University Srinagar - Jammu and Kashmir 190001, India Abstract: In this study, the multi-quasiparticle triaxial projected shell model (TPSM) is applied to investigate γ-vi- brational bands in transitional nuclei of 118−128Xe. We report that each triaxial intrinsic state has a γ-band built on it. The TPSM approach is evaluated by the comparison of TPSM results with available experimental data, which shows a satisfactory agreement. The energy ratios, B(E2) transition rates, and signature splitting of the γ-vibrational band are calculated. Keywords: triaxial projected shell model, triaxiality, yrast spectra, band diagram, back-bending, staggering, reduced transition probabilities DOI: 10.1088/1674-1137/44/7/074108 1 Introduction In the past few decades, the use of improved and sophisticated experimental techniques has made it pos- sible to provide sufficient data to describe the structure of The static and dynamic properties of a nucleus pre- nuclei in various mass regions of the nuclear chart. In dominantly dictate its shape or structure, and these prop- particular, the transitional nuclei around mass A ∼ 130 erties depend on the interactions among its constituents, have numerous interesting features, such as odd-even i.e, protons and neutrons. -

Mass Defect & Binding Energy the Nuclear Reaction Used by Stars To
Mass Defect & Binding Energy The nuclear reaction used by stars to produce energy (for most of their lives anyway) is the proton proton cycle: 1H + 1H → 2H + e– + ν 1H + 2H → 3He + γ 1H + 3He → 4He + e– + ν or 3He + 3He → 4He + 1H + 1H In the overall reaction, 4 hydrogen-1 atoms combine to form 1 He-4 atom plus some other parti- cles and energy. But examining the atomic masses of hydrogen and helium on the periodic table, the mass of four hydrogen atoms is greater than the mass of one helium atom. Subtracting the dif- ference gives: 4 × 1.007825 – 4.002603 = 0.028697 amu This difference is called the mass defect and measures the amount of “binding energy” stored in an atom’s nucleus or the amount of energy required to break up the nucleus back into the individ- ual protons and neutrons. In general, the mass defect is calculated by summing the mass of protons, neutrons, and electrons in an atom, and subtracting the atom’s actual atomic mass. The general formula is: Md = Z mp + N mn - Ma where Z is the atomic number, N is the number of neutrons in the atom, and Ma is the actual mea- sured mass of the atom. Placing Md into Einstein's equation for relating mass and energy gives the energy release from forming the atom from its constituent particles: 2 E = Md c where c is the speed of light (≈ 3.00 × 108 m/s). For example, using the information from the reac- tion above, one can deduce that to form 1 kg of 4He from requires 1.0073 kg of 1H. -

Mass Defect & Binding Energy
Sem-3 CC7 Nuclear Physics Mass Defect & Binding Energy According to nuclear particle experiments, the total mass of a nucleus (mnuc) is less than the sum of the masses of its constituent nucleons (protons and neutrons). For a nucleus with Z protons and mass number A, the mass difference or mass defect is given by Δm= Zmp + (A−Z)mn − mnuc where Zmp is the total mass of the protons, (A−Z)mn is the total mass of the neutrons, and mnuc is the mass of the nucleus. According to Einstein’s special theory of relativity, mass is a measure of the total energy of a system (E=mc2). The energy equivalent to mass defect is alled the binding energy B of the nucleus. Thus 2 B= [Zmp + (A−Z)mn − mnuc] c The binding energy is equal to the amount of energy released in forming the nucleus. Example 1. Calculate the mass defect and the binding energy of the deuteron. The mass of the −27 deuteron is mD=3.34359×10 kg or 2.014102u. Solution For the deuteron Z=1 and A=2. The mass defect for the deuteron is Δm=mp+mn−mD=1.008665 u+ 1.007825 u- 2.014102u = 0.002388u The binding energy of the deuteron is then B= Δm c2= Δm ×931.5 MeV/u=2.224MeV. Over two million electron volts are needed to break apart a deuteron into a proton and a neutron. This very large value indicates the great strength of the nuclear force. By comparison, the greatest amount of energy required to liberate an electron bound to a hydrogen atom by an attractive Coulomb force (an electromagnetic force) is about 10 eV. -

Iron-57 of Its Isotopes and Has an Sum of Protons + Neutrons 26 Fe Average Mass As Determined by a in the Nucleus
Elements - elements are pure homogeneous forms of matter Solid Liquid Gas Plamsa •constant volume & shape •constant volume but takes •varaible shape and volume •like a gas except it •very low compressibility on the shape of container that fills the container is composed of ions; •particles vibrate in place •low compressibility •high compressibility an ion is charged •highly ordered arrangement •random particle movement •complete freedom of motion atom or group of •do not flow or diffuse •moderate disorder •extreme disorder atoms. •strongest attractive forces •can flow and diffuse •flow and diffuse easily •examples: between particles •weaker attractive forces •weakest attractive forces - flames •generally more dense than •more dense than gases •least dense state - atmosphere of stars liquids •exert a pressure easily - a comet's tail Matter NO YES Pure Substance Can it be separated Mixture by physical means? Can it be decomposed by Is the mixture composition ordinary chemical means? identical throughout; uniform? NO YES YES NO element compound homogeneous heterogenous mixture mixture Co,Fe,S,H,O,C FeS, H2O, H2SO4 steel, air, blood, steam, wet iron, solution of sulfuric acid Cannot be separated Can be decomposed and rust on steel chemcially into simple chemcially separated Uniform appearance, Appearance, composition, substances into simple substances. composition and and properties are variable properties throughout in different parts of the sample Isotopes Is a mass number attached to the element? NO YES All atoms of the same element are not exactly alike mass number attached mass number equals the 57 The element is as a mixture also can be written as iron-57 of its isotopes and has an sum of protons + neutrons 26 Fe average mass as determined by a in the nucleus.