Towards Universal Access: Scaling up Priority HIV/AIDS Interventions in the Health Sector Preventing HIV Transmission from Mother to Child
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Page 1 2 0 0 7 a N N U a L R E P O R T
2007 ANNUAL REPORT IN SEARCH OF LASTING SOLUTIONS TO HIV/AIDS THE FOUNDATION FOR AIDS RESEARCH amfAR, THE FOUNDatION F O R A ID S R E S E ar C H , I S DEDICatED TO ENDING THE GLOBAL AIDS EPIDEMIC T H R O U G H I N N O V at I V E RESEARCH. Cover photos (from top): TREAT Asia Community Programs Manager Jennifer I-Ching Ho holds an HIV-positive child (photo: Karl Grobl); Dr. Nolwenn Jouvenet, Aaron Diamond AIDS Research Center (photo: Dr. Rowena Johnston); Shan Grant, Miguel Rivera, and Robert Green (photo: Winnie McCroy). FROM THE CHAIRMAN 02 A Global Force Against a Global Epidemic FROM THE CHIEF EXECUTIVE OFFICER 03 Good Research Drives Good Policy PEOPLE LIVING WITH HIV/AIDS 04 MAJOR ACCOMPLISHMENTS 06 RESEARCH 08 GLOBAL INITIATIVES 15 PUBLIC POLICY 22 EDUCATION AND INFORMATION 26 GIVING 30 Individual Giving Institutional Giving In-Kind Contributions Planned Giving Volunteer Support Workplace Giving FINANCIAL SUMMARY 44 From the Treasurer and the Chair of the Finance and Budget Committee Statement of Activities and Changes in Net Assets Statement of Financial Position LEADERSHIP AND ADVISORY COMMITTEES 46 ACKNOWLEDGMENTS FROM THE CHAIRMAN A Global FORCE AGAINST A Global EPIDEMIC Of the 33 million people living with HIV/AIDS, more than 90 percent are in the develop- ing world. For most of its 23 years at the leading edge of the fight against AIDS, amfAR has brought AIDS research, prevention, and education to countries hard hit by the epidemic. A global health threat demands nothing less than a global response. -

India's Trade News and Views
INDIA’S TRADE NEWS AND VIEWS 15 Oct to 31 Oct 2017 India rejects WTO push for new global e-commerce rules India has rejected fresh efforts by a clutch of countries led by the European Union (EU), Japan, Canada and Australia to negotiate new… Is globalization going to be unravelled? Globalization is getting very bad press in industrialized countries. From being touted aggressively only 10 years ago as a “win-win” development for all… India cautions against linking safeguard provisions to food security solutions India has cautioned against what it says are un-implementable transparency and safeguard provisions that are being sought… India flags concerns over linking food security steps with agri subsidies cut India has warned that a meeting of trade ministers in Buenos Aires in December will run into “serious consequences”—even “jeopardizing” the outcomes—if the European Union (EU), Brazil and other countries… RCEP trade ministers from India & other countries to meet in Manila in mid-November Trade ministers of 16 RCEP countries, including India and China, are expected to meet in Manila in mid- November to take… India to boost trade ties with Latin America, South East Asia India is looking to further diversify its trade to relatively newer markers in Latin America and Caribbean Islands… Keen on expanding bilateral trade with India: Minister Britain is keen on expanding its trade with India, a minister said today, asserting that there is scope to further strengthen… India-UK Future Tech Month to promote innovation ties The UK government's -

Number of Participants from Hospitals In
Exploratory study of the factors that influence nutrition interventions in the United Arab Emirates’ healthcare system Item Type Thesis Authors Algurg, Reem S.E.S. Rights <a rel="license" href="http://creativecommons.org/licenses/ by-nc-nd/3.0/"><img alt="Creative Commons License" style="border-width:0" src="http://i.creativecommons.org/l/by- nc-nd/3.0/88x31.png" /></a><br />The University of Bradford theses are licenced under a <a rel="license" href="http:// creativecommons.org/licenses/by-nc-nd/3.0/">Creative Commons Licence</a>. Download date 04/10/2021 14:53:45 Link to Item http://hdl.handle.net/10454/13964 University of Bradford eThesis This thesis is hosted in Bradford Scholars – The University of Bradford Open Access repository. Visit the repository for full metadata or to contact the repository team © University of Bradford. This work is licenced for reuse under a Creative Commons Licence. Exploratory study of the factors that influence nutrition interventions in the United Arab Emirates’ healthcare system Reem Saleh Easa Salah ALGURG Submitted for the degree of Doctor of Philosophy School of Management University of Bradford 2014 Abstract Reem Saleh Al Gurg Exploratory study of the factors that influence nutrition interventions in the United Arab Emirates’ healthcare system. INTRODUCTION: Non-communicable diseases are on the increase worldwide, causing more than 36 million deaths each year. Evidence of the link between the role of nutrition and reducing non-communicable diseases is predominant in the literature. The factors influencing intervention strategies/policies and activities, however, need attention. AIM: The study aims to examine the factors that influence nutrition interventions within the United Arab Emirates’ healthcare system. -

Discover the Power of Possibility at the Asian Business Leadership Forum™ Series ABLF ABLF
THE ABLF SERIES 2015 SHOW DECK Discover the power of possibility at the Asian Business Leadership Forum™ Series ABLF ABLF 2 3 ABLF THE ABLF SERIES IS DEDICATED TO THE VISIONARY LEADERS OF MODERN ASIA “ “ They say the sky is the limit for ambitions. We say: the sky is only the beginning. H.H. SHEIKH MOHAMMED BIN RASHID AL MAKTOUM Vice President and Prime Minister of the UAE and Ruler of Dubai 4 THis is NOT JUST THE MARKETING SHOW DECK OF ASIA’S MOST POWERFUL EVENT THIS IS YOUR CALL TO PLAY A ROLE IN RECALIBRATING THE DESTINY OF ASIA 2015 The Asian Business Leadership Forum (ABLF) Series is Asia’s premier leadership platform that convenes annually in the United Arab Emirates, with exclusive cameo showcases in Asia’s key business and economic centres. We bring together the continent’s finest leaders in cooperation, THE innovation and economic harmony. Recognising the exponential growth spurt of Asia today, the ABLF Series showcases the economic and intellectual capital of the region. Focused on creative business innovations, socio-economic transformation and, eventually, a globally successful Asia, it facilitates a meeting of minds of the ABLF best and brightest that Asia has to offer. At the heart of the ABLF Series lies our commitment to uphold excellence, integrity, innovation, SEriES sustainability, philanthropy and entrepreneurship. The ABLF Series embodies the very spirit of Asia, coming together in www.ablfseries.com celebration, conversation and collaboration 1 2 ABLF ABLF ARMANI HOTEL, BURJ KHALIFA, DUBAI 2014 ST. REGIS SAADIYAT ISLAND RESORT, ABU DHABI, AND DIFC GATE, DUBAI 2012 Since 2007, this global leadership platform has focused on Asia’s strengths through knowledge-based and information-sharing initiatives. -

Key Highlights
UNDER THE PATRONAGE OF H.H. SHEIKH NAHAYAN MABARAK AL-NAHAYAN MINISTER OF CULTURE, YOUTH AND COMMUNITY DEVELOPMENT, UAE KEY HIGHLIGHTS The Asian Business Leadership Forum (ABLF) Series is a powerhouse of ideas that facilitates creative business innovations for impactful socio-economic transformation Since 2007, this global leadership platform has focused on showcasing Asia’s economic and intellectual capital through knowledge-based and information-sharing initiatives that seek to build greater regional co-operation and expand the intra-Asia business corridors that promote economic development. In 2014, the ABLF Series unfolded as a two-day, closed-door, summit-style event, the ABLF Leadership Weekend, on December 13 and 14 at the Armani Hotel, Burj Khalifa, Dubai, UAE. Under the theme Asia Rising: Sustainable Leadership and Development in the 21st Century, it featured two key showcases: the Asian Business Leadership Forum and the ABLF Awards. www.ablfseries.com Pioneered, produced and presented by IEDEA • www.iedea.com DAY 1 DECEMBER 13, 2014 As the ABLF Series’ premier recognition platform, the ABLF Awards showcases leaders whose entrepreneurial brilliance is second only to their boundless sense of responsibility towards their communities and countries. The ABLF Grand Awards Ceremony represents the business zeitgeist of Asia and recognises excellence, innovation, good governance and best business practices in the region. This dynamic platform brings you face to face with Asia’s finest leaders, whose achievements, influences and initiatives have ensured that this century truly belongs to Asia. On December 13, 2014 – Day 1 of the ABLF Leadership Weekend – the ABLF Awards felicitated 12 Asian leaders of immense standing at a glittering gala, celebrating Asia’s leadership capital and inspiring millions of young Asian entrepreneurs around the world. -
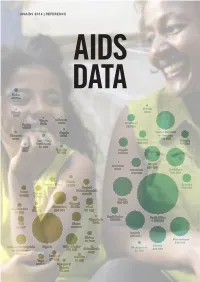
AIDS Data 2016
UNAIDS 2016 | REFERENCE AIDS DATA Sudan 26 000 Eritrea Djibouti 8900 5300 Yemen Lebanon Tunisia 3800 <500 Swaziland <1000 130 000 Algeria United Republic Morocco 4000 of Tanzania 8900 820 000 Iran (Islamic Namibia Rwanda Republic of) 130 000 120 000 Egypt 26 000 3500 Somalia Angola 15 000 190 000 Malawi Mauritius 580 000 2100 Botswana Zimbabwe 200 000 830 000 Liberia Burkina Faso 18 000 Lesotho 57 000 Central 180 000 Benin African Republic Guinea 69 000 40 000 71 000 Kenya Burundi 880 000 Niger Cameroon 46 000 Chad Sierra Leone 25 000 360 000 97 000 31 000 South Sudan South Africa Mauritania 100 000 4 100 000 Mali Ghana 7300 72 000 160 000 Uganda 840 000 Gabon Mozambique 31 000 890 000 Zambia Democratic Republic Nigeria Côte d’Ivoire Madagascar Cabo Verde 690 000 of the Congo 270 000 21 000 1700 220 000 Senegal Gambia 28 000 Togo 12 000 63 000 Equatorial Guinea 15 000 CONTENTS Introduction 2 Data summary 3 New HIV infections—all ages 10 New HIV infections—children aged 0–14 years 14 People living with HIV—all ages 18 People dying from AIDS-related illnesses 22 Pregnant women accessing medicines to prevent HIV transmission to their baby 26 People living with HIV on HIV treatment—adults 15 years and older 30 People living with HIV on HIV treatment—children aged 0–14 years 34 People living with HIV on HIV treatment—women and men 15 years and older 38 International assistance for the AIDS response 42 Domestic public and international expenditure for the AIDS response 55 Data collection and analysis methodology 72 INTRODUCTION The old saying “What gets measured gets done” may be a cliché, but is still very true for the response to HIV. -

India-UAE a KNOWLEDGE FORUM
India-UAE A KNOWLEDGE FORUM Exploring Opportunities Developing Strengths Overcoming Challenges May 24, 2010 - Abu Dhabi, UAE Under the Patronage of HH SHEIKH NAHAYAN MABARAK AL NAHAYAN Minister for Higher Education and Scientific Research, UAE INDIAN EXPRESSIONS proudly presents a prestigious knowledge forum India - UAE LEVERAGING THE KNOWLEDGE ECONOMY PARADIGM Exploring Opportunities • Developing Strengths • Overcoming Challenges in partnership with THE & RAJEN A. KILACHAND ™ KNOWLEDGEc FOUNDATION May 24, 2010 • Emirate Palace Hotel, Abu Dhabi, UAE AN INDIAN EXPRESSIONS EVENT © May 24, 2010 | Page 1 ‘No matter how many buildings, foundations, schools and hospitals we build, or how many bridges we raise, all these are material entities. The real spirit behind the progress is the human spirit, the able man with his intellect and capabilities’ ‘Father of the Nation’ HH Sheikh Zayed bin Sultan Al Nahyan Late President of the UAE AN INDIAN EXPRESSIONS EVENT © May 24, 2010 | Page 3 While his interests span areas as diverse as property development, banking, telecoms, automobiles, manufacturing, pharmaceuticals and tourism, HH Sheikh Nahayan is best known for his role in the UAE’s higher education sector. At the forefront of the knowledge institutions as part of his ministerial portfolio, Sheikh Nahayan is also at the helm of the UAE’s three government-sponsored centres of tertiary education - The United Arab Emirates University, Higher Colleges of Technology (HCT) and Zayed University. In addition, Sheikh Nahayan is the Chairman of CERT (Centre of Excellence for applied Research and Training), the commercial, research and training arm of HCT. HH SHEIKH NAHAYAN MABARAK AL NAHAYAN Minister for Higher Education and Scientific Research, UAE In attempting to fulfill the vision for knowledge-driven growth in the UAE, Sheikh Nahayan has always stressed that education revolves around building human capital through an inter-disciplinary approach. -

The Ablf Journey Through Our 10Th Anniversary Edition Show Deck for Promotional Purposes Only - Not Sale Or General Distribution
UNDER THE ROYAL PATRONAGE OF H.H. SHEIKH NAHAYAN MABARAK AL NAHAYAN CABINET MEMBER AND MINISTER OF TOLERANCE, UAE THE ABLF JOURNEY THROUGH OUR 10TH ANNIVERSARY EDITION SHOW DECK FOR PROMOTIONAL PURPOSES ONLY - NOT SALE OR GENERAL DISTRIBUTION. WWW.ABLF.COM WE ARE A MOVEMENT CELEBRATING WE REFLECT ASIA’S EVOLVING BUSINESS ENERGY 10 YEARS WE ARE THE CHRONICLERS, THE FAITHFUL STORYTELLERS OF SHOWCASING WE CONNECT THE CHANGE ARCHITECTS OF AN EXTRAORDINARY REGION ASIA’S WE HOST THE AMBASSADORS OF ASIA’S GROWTH NARRATIVES LEADERSHIP WE ARE THE ABLF EXCELLENCE ABLF SERIES UNDER THE ROYAL PATRONAGE OF H.H. SHEIKH NAHAYAN MABARAK AL NAHAYAN CABINET MEMBER AND MINISTER OF TOLERANCE, UAE PRESENTING THE ABLF AND THE UAE MINISTRY OF ECONOMY An Enduring Partnership With the official association of The Ministry of Economy, a vital arm of the UAE Ministry of Economy in 2016, the UAE Federal Government, plays a the Asian Business Leadership Forum key role in the sustainable development (ABLF) proudly commits the platform’s of the national economy. The Ministry future agenda to support the global supports the untiring efforts of the vision of the nation’s wise and ABLF in showcasing the UAE as a benevolent leadership. global destination that recognises and celebrates Asia’s finest leaders. IN ASSOCIATION WITH THE REGION'S MOST INFLUENTIAL LEADERSHIP PLATFORM THE ASIAN BUSINESS LEADERSHIP FORUM H.E. Sultan bin Saeed Al Mansouri, Minister of Economy, UAE, and H.H. Sheikh Ahmed bin Saeed Al Maktoum, Chairman and Chief Executive, Emirates Airline and Group, UAE; Chairman, Emirates NBD, UAE, at the ABLF Awards 2013 WWW.ABLF.COM THE EVOLVING FACE OF ASIA THE RISE OF FUTURE-FOCUSED LEADERSHIP 3 ABLF SERIES ABLF SERIES H.E. -

Communities at the Centre
COMMUNITIES AT THE CENTRE THE RESPONSE TO HIV IN MIDDLE EAST AND NORTH AFRICA GLOBAL AIDS UPDATE 2019 Cover photo credit: UNAIDS/P. Virot. CONTENTS MIDDLE EAST AND NORTH AFRICA 3 Algeria 14 Djibouti 16 Egypt 18 Iran (Islamic Republic of) 20 Jordan 22 Kuwait 24 Lebanon 26 Libya 28 Morocco 30 Oman 32 Qatar 34 Saudi Arabia 36 Somalia 38 Sudan 40 Syrian Arab Republic 42 Tunisia 44 Turkey 46 United Arab Emirates 48 Yemen 50 ANNEX ON METHODS 53 FIGURE 15.1 Number of people living with HIV who are not on antiretroviral therapy (aged 15 years and over), by sex, high-burden countries in Middle East and North Africa, 2018 Iran (Islamic Republic of) MIDDLE EAST AND NORTH AFRICA MIDDLE EAST AND NORTH Sudan Egypt Morocco Algeria 0 10 000 20 000 30 000 40 000 50 000 60 000 Number of people living with HIV who are not on antiretroviral therapy Řada1 Řada2 Males Females Source: UNAIDS 2019 estimates. 2 REGIONS IN FOCUS MIDDLE EAST AND NORTH AFRICA AT A GLANCE The epidemic in the Access to HIV testing, The increase in annual While some countries Middle East and North treatment and care in new infections is a have made progress Africa continues to grow, the region is well below sign that prevention in generating and with a 10% increase in the global average. Less programmes in many using timely strategic new infections and a 9% than half of people living countries are not information, increased increase in the annual with HIV are aware of reaching sufficient investment is needed number of AIDS-related their serostatus, and numbers of people at to fill large gaps in the deaths between 2010 treatment gaps among high risk of HIV infection. -

Political, Economic, and Health Determinants of Tuberculosis Incidence
University of Central Florida STARS Electronic Theses and Dissertations, 2004-2019 2016 Political, Economic, and Health Determinants of Tuberculosis Incidence Ashley Rutherford University of Central Florida Part of the Health Policy Commons, and the Health Services Administration Commons Find similar works at: https://stars.library.ucf.edu/etd University of Central Florida Libraries http://library.ucf.edu This Doctoral Dissertation (Open Access) is brought to you for free and open access by STARS. It has been accepted for inclusion in Electronic Theses and Dissertations, 2004-2019 by an authorized administrator of STARS. For more information, please contact [email protected]. STARS Citation Rutherford, Ashley, "Political, Economic, and Health Determinants of Tuberculosis Incidence" (2016). Electronic Theses and Dissertations, 2004-2019. 5645. https://stars.library.ucf.edu/etd/5645 POLITICAL, ECONOMIC, AND HEALTH DETERMINANTS OF TUBERCULOSIS INCIDENCE by ASHLEY E RUTHERFORD MPH University of South Carolina, 2013 B.S. Stetson University, 2012 A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the Department of Public Affairs in the College of Health and Public Affairs at the University of Central Florida Orlando, Florida Fall Term 2016 Major Professor: Lynn Unruh i © 2016 Ashley E Rutherford ii ABSTRACT The epidemiologic transition has shifted major causes of mortality from infectious disease to chronic disease; however, infectious diseases are again re-emerging as a major global concern (Diamond, 1997; Karlen, 1995; McNeil, 1976). This research aimed to identify potential areas of infectious disease influence that are not health-related in order to help governments and policymakers establish new policies, correct current policies, or further address these issues in order to effectively prevent and combat infectious disease. -

National Action Plan, for Combating Multidrug-Resistant Tuberculosis
FOR COMBATING MULTIDRUG-RESISTANT TUBERCULOSIS MULTIDRUG-RESISTANT FOR COMBATING ACTION PLAN NATIONAL YEAR TWO REPORT MAY 2018 TABLE OF CONTENTS ACRONYMS AND ABBREVIATIONS ACRONYMS AND ABBREVIATIONS .............................................3 INTRODUCTION ............................................................4 GOAL 1: STRENGTHEN DOMESTIC CAPACITY TO COMBAT MULTIDRUG-RESISTANT TUBERCULOSIS ....................................5 GOAL 2: IMPROVE INTERNATIONAL CAPACITY AND COLLABORATION TO COMBAT MULTIDRUG-RESISTANT TUBERCULOSIS ...........................7 GOAL 3: ACCELERATE BASIC AND APPLIED RESEARCH AND DEVELOPMENT TO COMBAT MULTIDRUG-RESISTANT TUBERCULOSIS .......................14 CONCLUSION .............................................................20 APPENDIX: MILESTONES AND ADVANCES .....................................21 Cover: USAID Trains Aeta Community Volunteers in TB Prevention. Jennifer belongs to the Aeta community - thought to be among the earliest inhabitants of the Philippines - in the mountains of Porac and Floridablanca, Pampanga. With limited access to health services, the 10,000 Aetas who live here are vulnerable to contracting TB. USAID trained Jennifer and other health volunteers to teach their community about TB and to serve as treatment partners for people with the disease. Photo by: Kdador/PBSP. Courtesy USAID Philippines. 2018 | NATIONAL ACTION PLAN FOR COMBATING MULTIDRUG-RESISTANT TUBERCULOSIS 2 ACRONYMS AND ABBREVIATIONS aDSM Active Drug Safety Management And MTB Mycobacterium tuberculosis Monitoring