On Borromean Links
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Borromean Rings: a Video About the New IMU Logo
The Borromean Rings: A Video about the New IMU Logo Charles Gunn and John M. Sullivan∗ Technische Universitat¨ Berlin Institut fur¨ Mathematik, MA 3–2 Str. des 17. Juni 136 10623 Berlin, Germany Email: {gunn,sullivan}@math.tu-berlin.de Abstract This paper describes our video The Borromean Rings: A new logo for the IMU, which was premiered at the opening ceremony of the last International Congress. The video explains some of the mathematics behind the logo of the In- ternational Mathematical Union, which is based on the tight configuration of the Borromean rings. This configuration has pyritohedral symmetry, so the video includes an exploration of this interesting symmetry group. Figure 1: The IMU logo depicts the tight config- Figure 2: A typical diagram for the Borromean uration of the Borromean rings. Its symmetry is rings uses three round circles, with alternating pyritohedral, as defined in Section 3. crossings. In the upper corners are diagrams for two other three-component Brunnian links. 1 The IMU Logo and the Borromean Rings In 2004, the International Mathematical Union (IMU), which had never had a logo, announced a competition to design one. The winning entry, shown in Figure 1, was designed by one of us (Sullivan) with help from Nancy Wrinkle. It depicts the Borromean rings, not in the usual diagram (Figure 2) but instead in their tight configuration, the shape they have when tied tight in thick rope. This IMU logo was unveiled at the opening ceremony of the International Congress of Mathematicians (ICM 2006) in Madrid. We were invited to produce a short video [10] about some of the mathematics behind the logo; it was shown at the opening and closing ceremonies, and can be viewed at www.isama.org/jms/Videos/imu/. -

A Knot-Vice's Guide to Untangling Knot Theory, Undergraduate
A Knot-vice’s Guide to Untangling Knot Theory Rebecca Hardenbrook Department of Mathematics University of Utah Rebecca Hardenbrook A Knot-vice’s Guide to Untangling Knot Theory 1 / 26 What is Not a Knot? Rebecca Hardenbrook A Knot-vice’s Guide to Untangling Knot Theory 2 / 26 What is a Knot? 2 A knot is an embedding of the circle in the Euclidean plane (R ). 3 Also defined as a closed, non-self-intersecting curve in R . 2 Represented by knot projections in R . Rebecca Hardenbrook A Knot-vice’s Guide to Untangling Knot Theory 3 / 26 Why Knots? Late nineteenth century chemists and physicists believed that a substance known as aether existed throughout all of space. Could knots represent the elements? Rebecca Hardenbrook A Knot-vice’s Guide to Untangling Knot Theory 4 / 26 Why Knots? Rebecca Hardenbrook A Knot-vice’s Guide to Untangling Knot Theory 5 / 26 Why Knots? Unfortunately, no. Nevertheless, mathematicians continued to study knots! Rebecca Hardenbrook A Knot-vice’s Guide to Untangling Knot Theory 6 / 26 Current Applications Natural knotting in DNA molecules (1980s). Credit: K. Kimura et al. (1999) Rebecca Hardenbrook A Knot-vice’s Guide to Untangling Knot Theory 7 / 26 Current Applications Chemical synthesis of knotted molecules – Dietrich-Buchecker and Sauvage (1988). Credit: J. Guo et al. (2010) Rebecca Hardenbrook A Knot-vice’s Guide to Untangling Knot Theory 8 / 26 Current Applications Use of lattice models, e.g. the Ising model (1925), and planar projection of knots to find a knot invariant via statistical mechanics. Credit: D. Chicherin, V.P. -

Gauss' Linking Number Revisited
October 18, 2011 9:17 WSPC/S0218-2165 134-JKTR S0218216511009261 Journal of Knot Theory and Its Ramifications Vol. 20, No. 10 (2011) 1325–1343 c World Scientific Publishing Company DOI: 10.1142/S0218216511009261 GAUSS’ LINKING NUMBER REVISITED RENZO L. RICCA∗ Department of Mathematics and Applications, University of Milano-Bicocca, Via Cozzi 53, 20125 Milano, Italy [email protected] BERNARDO NIPOTI Department of Mathematics “F. Casorati”, University of Pavia, Via Ferrata 1, 27100 Pavia, Italy Accepted 6 August 2010 ABSTRACT In this paper we provide a mathematical reconstruction of what might have been Gauss’ own derivation of the linking number of 1833, providing also an alternative, explicit proof of its modern interpretation in terms of degree, signed crossings and inter- section number. The reconstruction presented here is entirely based on an accurate study of Gauss’ own work on terrestrial magnetism. A brief discussion of a possibly indepen- dent derivation made by Maxwell in 1867 completes this reconstruction. Since the linking number interpretations in terms of degree, signed crossings and intersection index play such an important role in modern mathematical physics, we offer a direct proof of their equivalence. Explicit examples of its interpretation in terms of oriented area are also provided. Keywords: Linking number; potential; degree; signed crossings; intersection number; oriented area. Mathematics Subject Classification 2010: 57M25, 57M27, 78A25 1. Introduction The concept of linking number was introduced by Gauss in a brief note on his diary in 1833 (see Sec. 2 below), but no proof was given, neither of its derivation, nor of its topological meaning. Its derivation remained indeed a mystery. -
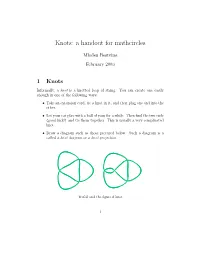
Knots: a Handout for Mathcircles
Knots: a handout for mathcircles Mladen Bestvina February 2003 1 Knots Informally, a knot is a knotted loop of string. You can create one easily enough in one of the following ways: • Take an extension cord, tie a knot in it, and then plug one end into the other. • Let your cat play with a ball of yarn for a while. Then find the two ends (good luck!) and tie them together. This is usually a very complicated knot. • Draw a diagram such as those pictured below. Such a diagram is a called a knot diagram or a knot projection. Trefoil and the figure 8 knot 1 The above two knots are the world's simplest knots. At the end of the handout you can see many more pictures of knots (from Robert Scharein's web site). The same picture contains many links as well. A link consists of several loops of string. Some links are so famous that they have names. For 2 2 3 example, 21 is the Hopf link, 51 is the Whitehead link, and 62 are the Bor- romean rings. They have the feature that individual strings (or components in mathematical parlance) are untangled (or unknotted) but you can't pull the strings apart without cutting. A bit of terminology: A crossing is a place where the knot crosses itself. The first number in knot's \name" is the number of crossings. Can you figure out the meaning of the other number(s)? 2 Reidemeister moves There are many knot diagrams representing the same knot. For example, both diagrams below represent the unknot. -

Computing the Writhing Number of a Polygonal Knot
Computing the Writhing Number of a Polygonal Knot ¡ ¡£¢ ¡ Pankaj K. Agarwal Herbert Edelsbrunner Yusu Wang Abstract Here the linking number, , is half the signed number of crossings between the two boundary curves of the ribbon, The writhing number measures the global geometry of a and the twisting number, , is half the average signed num- closed space curve or knot. We show that this measure is ber of local crossing between the two curves. The non-local related to the average winding number of its Gauss map. Us- crossings between the two curves correspond to crossings ing this relationship, we give an algorithm for computing the of the ribbon axis, which are counted by the writhing num- ¤ writhing number for a polygonal knot with edges in time ber, . A small subset of the mathematical literature on ¥§¦ ¨ roughly proportional to ¤ . We also implement a different, the subject can be found in [3, 20]. Besides the mathemat- simple algorithm and provide experimental evidence for its ical interest, the White Formula and the writhing number practical efficiency. have received attention both in physics and in biochemistry [17, 23, 26, 30]. For example, they are relevant in under- standing various geometric conformations we find for circu- 1 Introduction lar DNA in solution, as illustrated in Figure 1 taken from [7]. By representing DNA as a ribbon, the writhing number of its The writhing number is an attempt to capture the physical phenomenon that a cord tends to form loops and coils when it is twisted. We model the cord by a knot, which we define to be an oriented closed curve in three-dimensional space. -

A Remarkable 20-Crossing Tangle Shalom Eliahou, Jean Fromentin
A remarkable 20-crossing tangle Shalom Eliahou, Jean Fromentin To cite this version: Shalom Eliahou, Jean Fromentin. A remarkable 20-crossing tangle. 2016. hal-01382778v2 HAL Id: hal-01382778 https://hal.archives-ouvertes.fr/hal-01382778v2 Preprint submitted on 16 Jan 2017 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. A REMARKABLE 20-CROSSING TANGLE SHALOM ELIAHOU AND JEAN FROMENTIN Abstract. For any positive integer r, we exhibit a nontrivial knot Kr with r− r (20·2 1 +1) crossings whose Jones polynomial V (Kr) is equal to 1 modulo 2 . Our construction rests on a certain 20-crossing tangle T20 which is undetectable by the Kauffman bracket polynomial pair mod 2. 1. Introduction In [6], M. B. Thistlethwaite gave two 2–component links and one 3–component link which are nontrivial and yet have the same Jones polynomial as the corre- sponding unlink U 2 and U 3, respectively. These were the first known examples of nontrivial links undetectable by the Jones polynomial. Shortly thereafter, it was shown in [2] that, for any integer k ≥ 2, there exist infinitely many nontrivial k–component links whose Jones polynomial is equal to that of the k–component unlink U k. -

Forming the Borromean Rings out of Polygonal Unknots
Forming the Borromean Rings out of polygonal unknots Hugh Howards 1 Introduction The Borromean Rings in Figure 1 appear to be made out of circles, but a result of Freedman and Skora shows that this is an optical illusion (see [F] or [H]). The Borromean Rings are a special type of Brunninan Link: a link of n components is one which is not an unlink, but for which every sublink of n − 1 components is an unlink. There are an infinite number of distinct Brunnian links of n components for n ≥ 3, but the Borromean Rings are the most famous example. This fact that the Borromean Rings cannot be formed from circles often comes as a surprise, but then we come to the contrasting result that although it cannot be built out of circles, the Borromean Rings can be built out of convex curves, for example, one can form it from two circles and an ellipse. Although it is only one out of an infinite number of Brunnian links of three Figure 1: The Borromean Rings. 1 components, it is the only one which can be built out of convex compo- nents [H]. The convexity result is, in fact a bit stronger and shows that no 4 component Brunnian link can be made out of convex components and Davis generalizes this result to 5 components in [D]. While this shows it is in some sense hard to form most Brunnian links out of certain shapes, the Borromean Rings leave some flexibility. This leads to the question of what shapes can be used to form the Borromean Rings and the following surprising conjecture of Matthew Cook at the California Institute of Technology. -

Hyperbolic Structures from Link Diagrams
University of Tennessee, Knoxville TRACE: Tennessee Research and Creative Exchange Doctoral Dissertations Graduate School 5-2012 Hyperbolic Structures from Link Diagrams Anastasiia Tsvietkova [email protected] Follow this and additional works at: https://trace.tennessee.edu/utk_graddiss Part of the Geometry and Topology Commons Recommended Citation Tsvietkova, Anastasiia, "Hyperbolic Structures from Link Diagrams. " PhD diss., University of Tennessee, 2012. https://trace.tennessee.edu/utk_graddiss/1361 This Dissertation is brought to you for free and open access by the Graduate School at TRACE: Tennessee Research and Creative Exchange. It has been accepted for inclusion in Doctoral Dissertations by an authorized administrator of TRACE: Tennessee Research and Creative Exchange. For more information, please contact [email protected]. To the Graduate Council: I am submitting herewith a dissertation written by Anastasiia Tsvietkova entitled "Hyperbolic Structures from Link Diagrams." I have examined the final electronic copy of this dissertation for form and content and recommend that it be accepted in partial fulfillment of the equirr ements for the degree of Doctor of Philosophy, with a major in Mathematics. Morwen B. Thistlethwaite, Major Professor We have read this dissertation and recommend its acceptance: Conrad P. Plaut, James Conant, Michael Berry Accepted for the Council: Carolyn R. Hodges Vice Provost and Dean of the Graduate School (Original signatures are on file with official studentecor r ds.) Hyperbolic Structures from Link Diagrams A Dissertation Presented for the Doctor of Philosophy Degree The University of Tennessee, Knoxville Anastasiia Tsvietkova May 2012 Copyright ©2012 by Anastasiia Tsvietkova. All rights reserved. ii Acknowledgements I am deeply thankful to Morwen Thistlethwaite, whose thoughtful guidance and generous advice made this research possible. -

Arxiv:Math/9807161V1
FOUR OBSERVATIONS ON n-TRIVIALITY AND BRUNNIAN LINKS Theodore B. Stanford Mathematics Department United States Naval Academy 572 Holloway Road Annapolis, MD 21402 [email protected] Abstract. Brunnian links have been known for a long time in knot theory, whereas the idea of n-triviality is a recent innovation. We illustrate the relationship between the two concepts with four short theorems. In 1892, Brunn introduced some nontrivial links with the property that deleting any single component produces a trivial link. Such links are now called Brunnian links. (See Rolfsen [7]). Ohyama [5] introduced the idea of a link which can be independantly undone in n different ways. Here “undo” means to change some set of crossings to make the link trivial. “Independant” means that once you change the crossings in any one of the n sets, the link remains trivial no matter what you do to the other n − 1 sets of crossings. Philosophically, the ideas are similar because, after all, once you delete one component of a Brunnian link the result is trivial no matter what you do to the other components. We shall prove four theorems that make the relationship between Brunnian links and n-triviality more precise. We shall show (Theorem 1) that an n-component Brunnian link is (n − 1)-trivial; (Theorem 2) that an n-component Brunnian link with a homotopically trivial component is n-trivial; (Theorem 3) that an (n − k)-component link constructed from an n-component Brunnian link by twisting along k components is (n − 1)-trivial; and (Theorem 4) that a knot is (n − 1)-trivial if and only if it is “locally n-Brunnian equivalent” to the unknot. -

Splitting Numbers of Links
Splitting numbers of links Jae Choon Cha, Stefan Friedl, and Mark Powell Department of Mathematics, POSTECH, Pohang 790{784, Republic of Korea, and School of Mathematics, Korea Institute for Advanced Study, Seoul 130{722, Republic of Korea E-mail address: [email protected] Mathematisches Institut, Universit¨atzu K¨oln,50931 K¨oln,Germany E-mail address: [email protected] D´epartement de Math´ematiques,Universit´edu Qu´ebec `aMontr´eal,Montr´eal,QC, Canada E-mail address: [email protected] Abstract. The splitting number of a link is the minimal number of crossing changes between different components required, on any diagram, to convert it to a split link. We introduce new techniques to compute the splitting number, involving covering links and Alexander invariants. As an application, we completely determine the splitting numbers of links with 9 or fewer crossings. Also, with these techniques, we either reprove or improve upon the lower bounds for splitting numbers of links computed by J. Batson and C. Seed using Khovanov homology. 1. Introduction Any link in S3 can be converted to the split union of its component knots by a sequence of crossing changes between different components. Following J. Batson and C. Seed [BS13], we define the splitting number of a link L, denoted by sp(L), as the minimal number of crossing changes in such a sequence. We present two new techniques for obtaining lower bounds for the splitting number. The first approach uses covering links, and the second method arises from the multivariable Alexander polynomial of a link. Our general covering link theorem is stated as Theorem 3.2. -

Rock-Paper-Scissors Meets Borromean Rings
ROCK-PAPER-SCISSORS MEETS BORROMEAN RINGS MARC CHAMBERLAND AND EUGENE A. HERMAN 1. Introduction Directed graphs with an odd number of vertices n, where each vertex has both (n − 1)/2 incoming and outgoing edges, have a rich structure. We were lead to their study by both the Borromean rings and the game rock- paper-scissors. An interesting interplay between groups, graphs, topological links, and matrices reveals the structure of these objects, and for larger values of n, extensive computation produces some surprises. Perhaps most surprising is how few of the larger graphs have any symmetry and those with symmetry possess very little. In the final section, we dramatically sped up the computation by first computing a “profile” for each graph. 2. Three Weapons Let’s start with the two-player game rock-paper-scissors or RPS(3). The players simultaneously put their hands in one of three positions: rock (fist), paper (flat palm), or scissors (fist with the index and middle fingers sticking out). The winner of the game is decided as follows: paper covers rock, rock smashes scissors, and scissors cut paper. Mathematically, this game is referred to as a balanced tournament: with an odd number n of weapons, each weapon beats (n−1)/2 weapons and loses to the same number. This mutual dominance/submission connects RPS(3) with a seemingly disparate object: Borromean rings. Figure 1. Borromean Rings. 1 2 MARCCHAMBERLANDANDEUGENEA.HERMAN The Borromean rings in Figure 1 consist of three unknots in which the red ring lies on top of the blue ring, the blue on top of the green, and the green on top of the red. -

Matrix Integrals and Knot Theory 1
Matrix Integrals and Knot Theory 1 Matrix Integrals and Knot Theory Paul Zinn-Justin et Jean-Bernard Zuber math-ph/9904019 (Discr. Math. 2001) math-ph/0002020 (J.Kn.Th.Ramif. 2000) P. Z.-J. (math-ph/9910010, math-ph/0106005) J. L. Jacobsen & P. Z.-J. (math-ph/0102015, math-ph/0104009). math-ph/0303049 (J.Kn.Th.Ramif. 2004) G. Schaeffer and P. Z.-J., math-ph/0304034 P. Z.-J. and J.-B. Z., review article in preparation Carghjese, March 25, 2009 Matrix Integrals and Knot Theory 2 Courtesy of “U Rasaghiu” Matrix Integrals and Knot Theory 3 Main idea : Use combinatorial tools of Quantum Field Theory in Knot Theory Plan I Knot Theory : a few definitions II Matrix integrals and Link diagrams 1 2 4 dMeNtr( 2 M + gM ) N N matrices, N ∞ − × → RemovalsR of redundancies reproduces recent results of Sundberg & Thistlethwaite (1998) ⇒ based on Tutte (1963) III Virtual knots and links : counting and invariants Matrix Integrals and Knot Theory 4 Basics of Knot Theory a knot , links and tangles Equivalence up to isotopy Problem : Count topologically inequivalent knots, links and tangles Represent knots etc by their planar projection with minimal number of over/under-crossings Theorem Two projections represent the same knot/link iff they may be transformed into one another by a sequence of Reidemeister moves : ; ; Matrix Integrals and Knot Theory 5 Avoid redundancies by keeping only prime links (i.e. which cannot be factored) Consider the subclass of alternating knots, links and tangles, in which one meets alternatingly over- and under-crossings. For n 8 (resp.