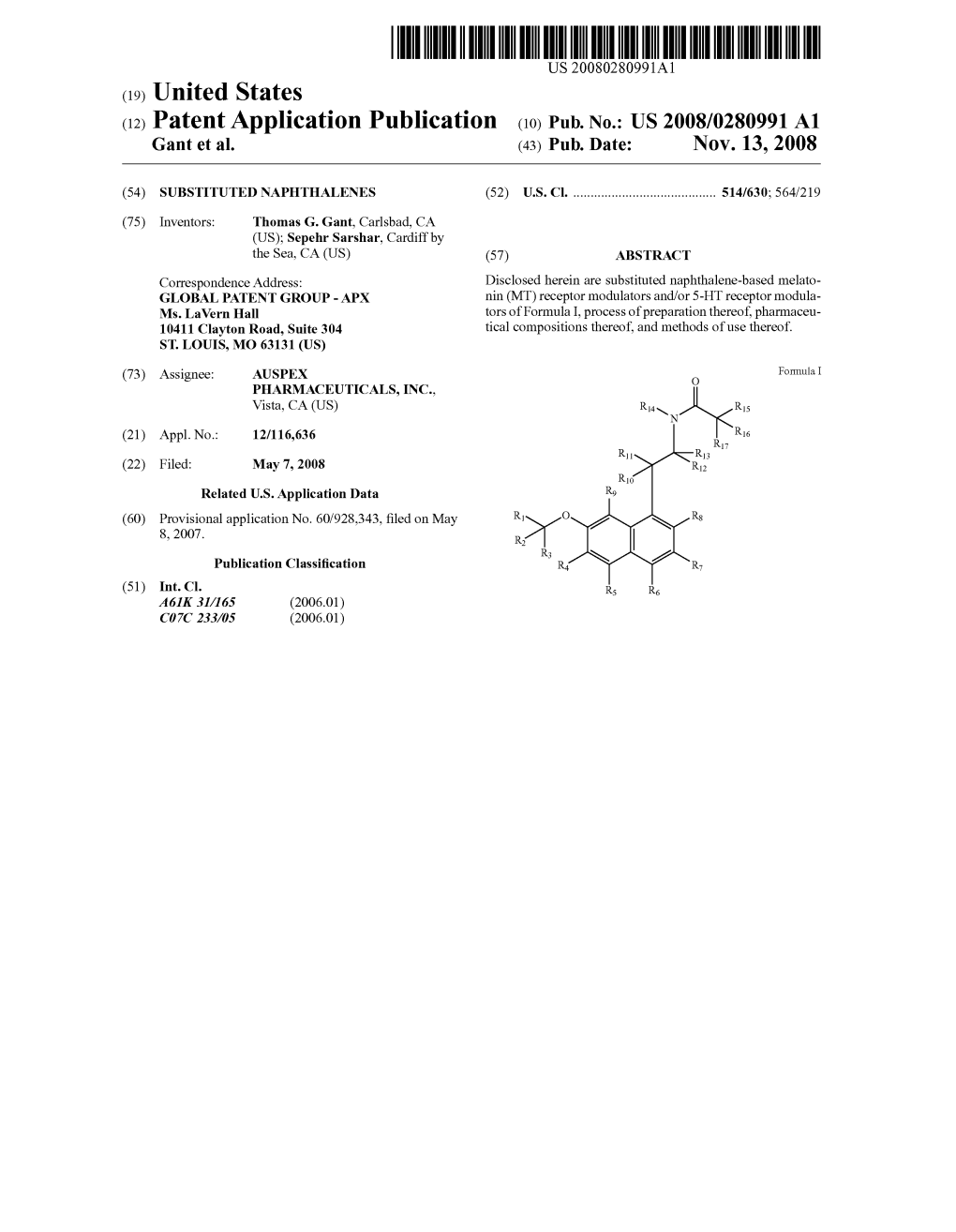
Us 2008/0280991 A1 8, 2007. Pdco
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Neuroprotective Effects of Melatonin: Possible Role in the Pathophysiology of Neuropsychiatric Disease
brain sciences Perspective The Neuroprotective Effects of Melatonin: Possible Role in the Pathophysiology of Neuropsychiatric Disease Jung Goo Lee 1,2 , Young Sup Woo 3, Sung Woo Park 2,4, Dae-Hyun Seog 5, Mi Kyoung Seo 6 and Won-Myong Bahk 3,* 1 Department of Psychiatry, College of Medicine, Haeundae Paik Hospital, Inje University, Busan 47392, Korea; [email protected] 2 Paik Institute for Clinical Research, Department of Health Science and Technology, Graduate School, Inje University, Busan 47392, Korea; [email protected] 3 Department of Psychiatry, College of Medicine, The Catholic University of Korea, Seoul 07345, Korea; [email protected] 4 Department of Convergence Biomedical Science, College of Medicine, Inje University, Busan 47392, Korea 5 Department of Biochemistry, College of Medicine, Inje University, Busan 47392, Korea; [email protected] 6 Paik Institute for Clinical Research, Inje University, Busan 47392, Korea; [email protected] * Correspondence: [email protected] Received: 16 September 2019; Accepted: 19 October 2019; Published: 21 October 2019 Abstract: Melatonin is a hormone that is secreted by the pineal gland. To date, melatonin is known to regulate the sleep cycle by controlling the circadian rhythm. However, recent advances in neuroscience and molecular biology have led to the discovery of new actions and effects of melatonin. In recent studies, melatonin was shown to have antioxidant activity and, possibly, to affect the development of Alzheimer’s disease (AD). In addition, melatonin has neuroprotective effects and affects neuroplasticity, thus indicating potential antidepressant properties. In the present review, the new functions of melatonin are summarized and a therapeutic target for the development of new drugs based on the mechanism of action of melatonin is proposed. -

Pharmacometabolomics of Response to Sertraline and to Placebo in Major Depressive Disorder – Possible Role for Methoxyindole Pathway
Pharmacometabolomics of Response to Sertraline and to Placebo in Major Depressive Disorder – Possible Role for Methoxyindole Pathway Hongjie Zhu1., Mikhail B. Bogdanov2., Stephen H. Boyle1, Wayne Matson3, Swati Sharma3, Samantha Matson3,4, Erik Churchill1, Oliver Fiehn5, John A. Rush6, Ranga R. Krishnan1,6, Eve Pickering7, Marielle Delnomdedieu8, Rima Kaddurah-Daouk1*, Pharmacometabolomics Research Network 1 Department of Psychiatry and Behavioral Sciences, Duke University, Durham, North Carolina, United States of America, 2 Department of Neurology and Neuroscience Weill Cornell Medical College, New York, New York, United States of America, 3 Department of Systems Biochemistry, Bedford VA Medical Center, Bedford, Massachusetts, United States of America, 4 Massachusetts General Hospital, Boston, Massachusetts, United States of America, 5 Genome Center, University of California Davis, Davis, California, United States of America, 6 Duke-NUS Graduate Medical School, Singapore, Singapore, 7 Pfizer Global R&D, Clinical Research Statistics, Groton, Connecticut, United States of America, 8 Pfizer Global R&D, Neuroscience Clinical Research, Groton, Connecticut, United States of America Abstract Therapeutic response to selective serotonin (5-HT) reuptake inhibitors in Major Depressive Disorder (MDD) varies considerably among patients, and the onset of antidepressant therapeutic action is delayed until after 2 to 4 weeks of treatment. The objective of this study was to analyze changes within methoxyindole and kynurenine (KYN) branches of tryptophan pathway to determine whether differential regulation within these branches may contribute to mechanism of variation in response to treatment. Metabolomics approach was used to characterize early biochemical changes in tryptophan pathway and correlated biochemical changes with treatment outcome. Outpatients with MDD were randomly assigned to sertraline (n = 35) or placebo (n = 40) in a double-blind 4-week trial; response to treatment was measured using the 17-item Hamilton Rating Scale for Depression (HAMD17). -

Drug Repurposing for the Management of Depression: Where Do We Stand Currently?
life Review Drug Repurposing for the Management of Depression: Where Do We Stand Currently? Hosna Mohammad Sadeghi 1,†, Ida Adeli 1,† , Taraneh Mousavi 1,2, Marzieh Daniali 1,2, Shekoufeh Nikfar 3,4,5 and Mohammad Abdollahi 1,2,* 1 Toxicology and Diseases Group (TDG), Pharmaceutical Sciences Research Center (PSRC), The Institute of Pharmaceutical Sciences (TIPS), Tehran University of Medical Sciences, Tehran 1417614411, Iran; [email protected] (H.M.S.); [email protected] (I.A.); [email protected] (T.M.); [email protected] (M.D.) 2 Department of Toxicology and Pharmacology, School of Pharmacy, Tehran University of Medical Sciences, Tehran 1417614411, Iran 3 Personalized Medicine Research Center, Endocrinology and Metabolism Research Institute, Tehran University of Medical Sciences, Tehran 1417614411, Iran; [email protected] 4 Pharmaceutical Sciences Research Center (PSRC) and the Pharmaceutical Management and Economics Research Center (PMERC), Evidence-Based Evaluation of Cost-Effectiveness and Clinical Outcomes Group, The Institute of Pharmaceutical Sciences (TIPS), Tehran University of Medical Sciences, Tehran 1417614411, Iran 5 Department of Pharmacoeconomics and Pharmaceutical Administration, School of Pharmacy, Tehran University of Medical Sciences, Tehran 1417614411, Iran * Correspondence: [email protected] † Equally contributed as first authors. Citation: Mohammad Sadeghi, H.; Abstract: A slow rate of new drug discovery and higher costs of new drug development attracted Adeli, I.; Mousavi, T.; Daniali, M.; the attention of scientists and physicians for the repurposing and repositioning of old medications. Nikfar, S.; Abdollahi, M. Drug Experimental studies and off-label use of drugs have helped drive data for further studies of ap- Repurposing for the Management of proving these medications. -

Npp200972.Pdf
Neuropsychopharmacology (2009) 34, 2390–2403 & 2009 Nature Publishing Group All rights reserved 0893-133X/09 $32.00 www.neuropsychopharmacology.org Mechanisms Contributing to the Phase-Dependent Regulation of Neurogenesis by the Novel Antidepressant, Agomelatine, in the Adult Rat Hippocampus Ame´lie Soumier1, Mounira Banasr1, Sylviane Lortet1, Fre´de´rique Masmejean1, Nathalie Bernard1, Lydia Kerkerian-Le-Goff1, Cecilia Gabriel2, Mark J Millan3, Elisabeth Mocaer2 and Annie Daszuta*,1 1 2 3 IC2N, IBDML, UMR 6216, Marseille, France; IRIS, Courbevoie, France; IDR Servier, Croissy-sur-Seine, France Agomelatine is a novel antidepressant acting as a melatonergic receptor agonist and serotonergic (5-HT ) receptor antagonist. In adult 2C rats, chronic agomelatine treatment enhanced cell proliferation and neurogenesis in the ventral hippocampus (VH), a region pertinent to mood disorders. This study compared the effects of agomelatine on cell proliferation, maturation, and survival and investigated the cellular mechanisms underlying these effects. Agomelatine increased the ratio of mature vs immature neurons and enhanced neurite outgrowth of granular cells, suggesting an acceleration of maturation. The influence of agomelatine on maturation and survival was accompanied by a selective increase in the levels of BDNF (brain-derived neurotrophic factor) vs those of VEGF (vascular endothelial factor) and IGF-1 (insulin-like growth factor 1), which were not affected. Agomelatine also activated several cellular signals (extracellular signal-regulated kinase1/2, protein kinase B, and glycogen synthase kinase 3b) known to be modulated by antidepressants and implicated in the control of proliferation/survival. Furthermore, as agomelatine possesses both melatonergic agonist and serotonergic (5-HT2C) antagonist properties, we determined whether melatonin and 5-HT2C receptor antagonists similarly influence cell proliferation and survival. -

Association of Selective Serotonin Reuptake Inhibitors with the Risk for Spontaneous Intracranial Hemorrhage
Supplementary Online Content Renoux C, Vahey S, Dell’Aniello S, Boivin J-F. Association of selective serotonin reuptake inhibitors with the risk for spontaneous intracranial hemorrhage. JAMA Neurol. Published online December 5, 2016. doi:10.1001/jamaneurol.2016.4529 eMethods 1. List of Antidepressants for Cohort Entry eMethods 2. List of Antidepressants According to the Degree of Serotonin Reuptake Inhibition eMethods 3. Potential Confounding Variables Included in Multivariate Models eMethods 4. Sensitivity Analyses eFigure. Flowchart of Incident Antidepressant (AD) Cohort Definition and Case- Control Selection eTable 1. Crude and Adjusted Rate Ratios of Intracerebral Hemorrhage Associated With Current Use of SSRIs Relative to TCAs eTable 2. Crude and Adjusted Rate Ratios of Subarachnoid Hemorrhage Associated With Current Use of SSRIs Relative to TCAs eTable 3. Crude and Adjusted Rate Ratios of Intracranial Extracerebral Hemorrhage Associated With Current Use of SSRIs Relative to TCAs. eTable 4. Crude and Adjusted Rate Ratios of Intracerebral Hemorrhage Associated With Current Use of Antidepressants With Strong Degree of Inhibition of Serotonin Reuptake Relative to Weak eTable 5. Crude and Adjusted Rate Ratios of Subarachnoid Hemorrhage Associated With Current Use of Antidepressants With Strong Degree of Inhibition of Serotonin Reuptake Relative to Weak eTable 6. Crude and Adjusted Rate Ratios of Intracranial Extracerebral Hemorrhage Associated With Current Use of Antidepressants With Strong Degree of Inhibition of Serotonin Reuptake Relative to Weak This supplementary material has been provided by the authors to give readers additional information about their work. © 2016 American Medical Association. All rights reserved. Downloaded From: https://jamanetwork.com/ on 10/02/2021 eMethods 1. -

A Study on the Effects of Agomelatine on Food Intake and Body Weight in Restraint Stress Model in Adult Swiss Albino Mice
Online - 2455-3891 Vol 10, Issue 9, 2017 Print - 0974-2441 Research Article A STUDY ON THE EFFECTS OF AGOMELATINE ON FOOD INTAKE AND BODY WEIGHT IN RESTRAINT STRESS MODEL IN ADULT SWISS ALBINO MICE SHANMUGAPRIYA S*, NIVEDHITHAA S, BHUVANESWARI K Department of Pharmacology, PSG Institute of Medical Sciences and Research, Peelamedu, Coimbatore, Tamil Nadu, India. Email: [email protected] Received: 01 May 2017, Revised and Accepted: 25 May 2017 ABSTRACT Objectives: Agomelatine is a novel melatonin (MT) receptor agonist at MT 1 and 2, serotonin receptor antagonist and an effective chronobiotic agent. The study was designed to evaluate the effects of agomelatine on body weight and food intake in restraint stress model in adult Swiss albino mice. Methods: After the approval of Institutional Animal Ethics Committee, 40 male Swiss albino mice were randomly divided into four groups of 10 animals each; two were treatment groups which received 25 mg/kg (low dose) agomelatine, 50 mg/kg (high dose) agomelatine, standard group given trazodone and the control group administered the vehicle (1% hydroxyethyl cellulose [HEC]) intraperitoneally for the last 14 days in the 3 weeks study period. Chronic restraint stress was given for 4 hrs per day for all groups starting from day 0 to 21. Results: Using paired t-test, both 12 hrs (p=0.011) and 24 hrs (p<0.001) food intake in the high dose agomelatine group were significantly increased. Between groups using ANOVA test showed a statistically significant increase in food intake for this group when compared to the control group. Unlike the low dose agomelatine group (p=0.205), the mean body weight in the group treated with high dose agomelatine revealed a statistically significant rise compared to that of the control (p=0.001) in ANOVA test. -

New-Medicine-Report-Agomelatine
Suffolk Mental Health Partnership NHS Trust Drug & Therapeutics Committee New Medicine Report Medicine Agomelatine (Valdoxan, Servier) Document Agreed at Suffolk D&T 18th February 2009 status Date of last 22nd July 2009 revision Traffic light Double Red - Prescribing not supported in either general practice or decision secondary/tertiary care Prescribers 5 - Judgement reserved rating Mechanism Agomelatine is a synthetic analogue of the hormone melatonin, and strongly of action binds to and stimulates the activity of melatonin MT1 and MT2 receptors, normalising disturbed circadian rhythms and disrupted sleep-wake cycles. (Melatonin regulates circadian rhythms, including sleep-wake cycles.) Disturbances in circadian rhythms have been implemented in the development of mood disorders. Agomelatine is also a serotonin-receptor antagonist and binds to and inhibits the activity of serotonin 5HT-2C receptors. This antagonism is associated with antidepressant and anti-anxiety activity, and increases slow-wave sleep. Agomelatine does not affect the uptake of serotonin, noradrenaline or dopamine. The inhibition of 5HT-2C receptors increases noradrenaline and dopamine in the frontal cortex and may contribute to agomelatine’s antidepressant activity. Indication Treatment of major depressive disorders in adults Dosage 25mg increasing to 50mg daily Treatment SSRIs alternatives Review prepared by Katie Smith, Director, East Anglia Medicines Information Service May be freely copied by NHS agencies, not to be used for promotional purposes This is an SMHPT -

Australian Public Assessment Report for Agomelatine
Australian Public Assessment Report for Agomelatine Proprietary Product Name: Valdoxan Submission No: PM-2009-00483-3-1 Sponsor: Servier Laboratories (Australia) Pty Ltd October 2010 Contents I. Introduction to Product Submission........................................................................3 Submission Details.................................................................................................................. 3 Product Background................................................................................................................ 3 Regulatory Status .................................................................................................................... 5 Product Information ................................................................................................................ 5 II. Quality Findings.........................................................................................................6 Introduction............................................................................................................................. 6 Drug Product........................................................................................................................... 6 Bioavailability......................................................................................................................... 6 Quality Summary and Conclusions......................................................................................... 7 III. Nonclinical Findings ..................................................................................................7 -

The Role of Adaptogens and Nervines in Clinical Practice
The Role of Selected Adaptogens and Nervines in Clinical Practice Tieraona Low Dog, MD Chair: US Pharmacopeia Dietary Supplements Admissions Joint Standard Setting Sub-Committee Author of National Geographic’s Fortify Your Life, Healthy At Home, Life Is Your Best Medicine Guide to Medicinal Herbs Copyright © Integrative Medicine Concept, LLC. All Rights Reserved. Disclosure • Tieraona Low Dog, MD has the following to disclose: • Health Advisory Board: Pharmaca • Director of Scientific & Regulatory Affairs: Healthy Lifestyle Brands • Consultant/Spokesperson: FoodState • This talk will not discuss off-label and/or investigational use of pharmaceuticals or devices not yet approved by the FDA. Copyright © Integrative Medicine Concept, LLC. All Rights Reserved. Stress in the 21st Century • 75-90% of all physician’s office visits are for stress-related conditions/complaints. • Lifetime prevalence of emotional disorder > 50%. • 43% of all adults suffer adverse health effects from stress. • Stress contributes to obesity, diabetes, heart disease, high blood pressure, depression, anxiety, asthma, irritable bowel disease, infertility, headaches, muscle tension and premature aging. National Institute of Mental Health: "Fact Sheet on Stress." American Heart Association: "How Does Stress Affect You." Mayo Clinic: "Stress: Constant stress puts your health at risk.” Copyright © Integrative Medicine Concept, LLC. All Rights Reserved. Trends in prescriptions of major classes of psychiatric drugs 1998–2010. Stephen Ilyas, and Joanna Moncrieff BJP 2012;200:393-398 -

The Effortless Sleep Method: Cure for Insomnia
Sleeping Pills: An Introduction 1 1 Sleeping Pills: An Introduction What Are Sleeping Pills? Most sleeping pills are “sedative hypnotics.” That’s a specific class of drugs used to induce and/or maintain sleep. Sedative hypnotics include benzodiazepines, barbiturates, and various hypnotics. SLEEPING PILL ABUSE: ITS SIGNS AND SYMPTOMS A number of people underestimate the powerful grip sleeping pills like Ambien or Sonata can have over someone’s life and the dangers of abusing these drugs. 2 The Effortless Sleep Method: Cure for Insomnia... Many people abusing sleeping pills experience memory and concentration problems. Some of the signs of sleeping pill abuse include: • Slurred speech • Uncoordinated movements • Unsteady gait • Inability to focus • Impaired memory • Unusual euphoria. SLEEPING PILLS: ITS MAIN DANGERS Both the immediate and long-term dangers of sleeping pill abuse are enough for most people to exercise caution when using them. However, many people aren’t aware of the dangers of these medications. The dangerous effects of sleep medications range from seizures to depressed breathing. Some people also experience allergic reactions from sleeping pills that can cause difficulty breathing, chest pain, nausea and swelling. Though rare, people who use sleeping pills may even develop parasomnias. Parasomnias are defined as sleep disorders that include behaviors like sleep-walking, sleep-eating, sleep-sex, sleep- driving and other potentially dangerous sleep-related activities. The immediate dangers of sleeping pills range from minor fatigue to coma. Some of these side effects can even lead to deadly overdoses, casting light on the true dangers of sleeping pills. Common symptoms of sleeping pill abuse are: • Dizziness • Dry mouth • Difficulty with coordination • Daytime drowsiness • Memory loss • Unusual dreams • Itching and swelling Sleeping Pills: An Introduction 3 • Lightheadedness • Depressed breathing rate. -

Agomelatine Softens Depressive-Like Behavior Through the Regulation of Autophagy and Apoptosis
Hindawi BioMed Research International Volume 2021, Article ID 6664591, 10 pages https://doi.org/10.1155/2021/6664591 Research Article Agomelatine Softens Depressive-Like Behavior through the Regulation of Autophagy and Apoptosis Fengpei Chen,1 Shijia Chen ,2 Jie Liu,1 Nashwa Amin,2,3 Weidong Jin ,1,4 and Marong Fang 2 1The Second Clinical Medical College of Zhejiang Chinese Medical University, Hangzhou 310053, China 2Institute of Neuroscience, Zhejiang University School of Medicine, Hangzhou 310058, China 3Department of Zoology, Faculty of Science, Aswan University, Egypt 4Zhejiang Mental Health Center, Tongde Hospital of Zhejiang province, Hangzhou 310012, China Correspondence should be addressed to Weidong Jin; [email protected] and Marong Fang; [email protected] Received 12 December 2020; Revised 11 January 2021; Accepted 26 February 2021; Published 18 March 2021 Academic Editor: Andrea Scribante Copyright © 2021 Fengpei Chen et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Depression is a common and disabling mental disorder with high recurrence rate. Searching for more effective treatments for depression is a long-standing primary objective in neuroscience. Agomelatine (AGO) was reported as an antidepressant with unique pharmacological effects. However, its effects and the underlying mechanism are still unclear. In this study, we sought to evaluate the antidepressant effects of AGO on the chronic restraint stress (CRS) mouse model and preliminarily investigate its effects on the gut microbial metabolites. The CRS model mice were established in 28 days with AGO (60 mg/kg/day, by oral) or fluoxetine (15 mg/kg/day, by oral) administration. -

VALDOXAN® 25Mg Agomelatine (Pronounced A-Go-Mel-A-Tin)
VALDOXAN® 25mg Agomelatine (pronounced a-go-mel-a-tin) Consumer Medicine Information WHAT IS IN THIS positive benefits on mood and sleep • you are currently taking LEAFLET in depression. fluvoxamine (a drug used in the VALDOXAN is not addictive. treatment of depression) or ciprofloxacin (an antibiotic This leaflet answers some common VALDOXAN does not cause used to treat infections) questions about VALDOXAN. It discontinuation symptoms and can be does not contain all the available stopped without the need to taper • you have an allergy to information. You can obtain more dose. VALDOXAN or any of the information about VALDOXAN by ingredients (including lactose) In clinical studies VALDOXAN had talking to your doctor or pharmacist. listed at the end of this leaflet no effect on sexual function. All medications have risks and • the packaging is torn or shows VALDOXAN is not recommended benefits. Your doctor has weighed signs of tampering for children, adolescents (under 18 the risks of you taking VALDOXAN years old) or elderly patients aged 75 • the expiry date (EXP) printed against the benefits this medicine is or older. on the pack has passed. expected to have for you. Your doctor may prescribe If you have any concerns about Before you start to take VALDOXAN for another purpose. taking this medicine, ask your VALDOXAN Ask your doctor if you have any doctor or pharmacist. Keep this questions about why VALDOXAN A routine blood test should be leaflet with the medicine. has been prescribed for you. performed before treatment to You may need to read it again. check how your liver is functioning.