Acute Toxicity of Cyanide in Aerobic Respiration: Theoretical and Experimental Support for Murburn Explanation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Hazardous Chemicals in Secondhand Marijuana Smoke
Hazardous Chemicals in Secondhand Marijuana Smoke “The following 33 marijuana smoke constituents included in Table 1 are listed under 33 Chemicals Proposition 65 as causing cancer: acetaldehyde, acetamide, acrylonitrile, 4- aminobiphenyl, arsenic, benz[a]anthracene, benzene, benzo[a]pyrene, That Can benzo[b]fluoranthene, benzo[j]fluoranthene, benzo[k]fluoranthene, benzofuran, 1,3- butadiene, cadmium, carbazole, catechol, chromium (hexavalent compounds), Cancer chrysene, dibenz[a,h]anthracene, dibenz[a,i]pyrene, dibenzo[a,e]pyrene, “Many of the chemical diethylnitrosamine, dimethylnitrosamine, formaldehyde, indeno[1,2,3,-c,d]pyrene, constituents that have been isoprene, lead, mercury, 5-methylchrysene, naphthalene, nickel, pyridine, and identified in marijuana smoke quinoline.” are carcinogens.” 2009 OEHHA document, Evidence on the Carcinogenicity of Marijuana Smoke Hydrogen Cyanide interferes with the normal use of oxygen by nearly every organ of Hydrogen the body. Exposure to hydrogen cyanide (AC) can be rapidly fatal. It has whole-body (systemic) effects, particularly affecting those organ systems most sensitive to low Cyanide oxygen levels: the central nervous system (brain), the cardiovascular system (heart Is the same chemical used for and blood vessels), and the pulmonary system (lungs). Hydrogen cyanide (AC) is a chemical weapons. chemical warfare agent (military designation, AC). Ammonia gas is a severe respiratory tract irritant. Can cause severe irritation of the Ammonia nose and throat. Can cause life-threatening accumulation of fluid in the lungs Household cleaner used on (pulmonary edema). Symptoms may include coughing, shortness of breath, difficult floors and toilets. There is 3 breathing and tightness in the chest. Symptoms may develop hours after exposure times more in secondhand and are made worse by physical effort. -

Pharmacy and Poisons (Third and Fourth Schedule Amendment) Order 2017
Q UO N T FA R U T A F E BERMUDA PHARMACY AND POISONS (THIRD AND FOURTH SCHEDULE AMENDMENT) ORDER 2017 BR 111 / 2017 The Minister responsible for health, in exercise of the power conferred by section 48A(1) of the Pharmacy and Poisons Act 1979, makes the following Order: Citation 1 This Order may be cited as the Pharmacy and Poisons (Third and Fourth Schedule Amendment) Order 2017. Repeals and replaces the Third and Fourth Schedule of the Pharmacy and Poisons Act 1979 2 The Third and Fourth Schedules to the Pharmacy and Poisons Act 1979 are repealed and replaced with— “THIRD SCHEDULE (Sections 25(6); 27(1))) DRUGS OBTAINABLE ONLY ON PRESCRIPTION EXCEPT WHERE SPECIFIED IN THE FOURTH SCHEDULE (PART I AND PART II) Note: The following annotations used in this Schedule have the following meanings: md (maximum dose) i.e. the maximum quantity of the substance contained in the amount of a medicinal product which is recommended to be taken or administered at any one time. 1 PHARMACY AND POISONS (THIRD AND FOURTH SCHEDULE AMENDMENT) ORDER 2017 mdd (maximum daily dose) i.e. the maximum quantity of the substance that is contained in the amount of a medicinal product which is recommended to be taken or administered in any period of 24 hours. mg milligram ms (maximum strength) i.e. either or, if so specified, both of the following: (a) the maximum quantity of the substance by weight or volume that is contained in the dosage unit of a medicinal product; or (b) the maximum percentage of the substance contained in a medicinal product calculated in terms of w/w, w/v, v/w, or v/v, as appropriate. -
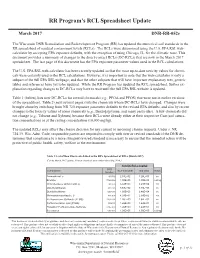
RR Program's RCL Spreadsheet Update
RR Program’s RCL Spreadsheet Update March 2017 RR Program RCL Spreadsheet Update DNR-RR-052e The Wisconsin DNR Remediation and Redevelopment Program (RR) has updated the numerical soil standards in the August 2015 DNR-RR- 052b RR spreadsheet of residual contaminant levels (RCLs). The RCLs were determined using the U.S. EPA RSL web- calculator by accepting EPA exposure defaults, with the exception of using Chicago, IL, for the climatic zone. This documentThe U.S. provides EPA updateda summary its Regionalof changes Screening to the direct-contact Level (RSL) RCLs website (DC-RCLs) in June that2015. are To now reflect in the that March 2017 spreadsheet.update, the The Wisconsin last page ofDNR this updated document the has numerical the EPA exposuresoil standards, parameter or residual values usedcontaminant in the RCL levels calculations. (RCLs), in the Remediation and Redevelopment program’s spreadsheet of RCLs. This document The providesU.S. EPA a RSL summary web-calculator of the updates has been incorporated recently updated in the Julyso that 2015 the spreadsheet.most up-to-date There toxicity were values no changes for chemi - cals madewere certainlyto the groundwater used in the RCLs,RCL calculations. but there are However, many changes it is important in the industrial to note that and the non-industrial web-calculator direct is only a subpartcontact of the (DC) full RCLsEPA RSL worksheets. webpage, Tables and that 1 andthe other 2 of thissubparts document that will summarize have important the DC-RCL explanatory changes text, generic tablesfrom and the references previous have spreadsheet yet to be (Januaryupdated. -
(12) Patent Application Publication (10) Pub. No.: US 2013/0059868 A1 Miner Et Al
US 2013 0059868A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2013/0059868 A1 Miner et al. (43) Pub. Date: Mar. 7, 2013 (54) TREATMENT OF GOUT Publication Classification (75) Inventors: Jeffrey Miner, San Diego, CA (US); Jean-Luc Girardet, San Diego, CA (51) Int. Cl. (US); Barry D. Quart, Encinitas, CA A613 L/496 (2006.01) (US) A6IP 29/00 (2006.01) (73) Assignee: Ardea Biociences, Inc., San Diego, CA A6IP 9/06 (2006.01) (US) A613 L/426 (2006.01) A 6LX3/59 (2006.01) (21) Appl. No.: 13/637,343 (52) U.S. Cl. ...................... 514/262.1: 514/384: 514/365 (22) PCT Fled: Mar. 29, 2011 (86) PCT NO.: PCT/US11A3O364 (57) ABSTRACT S371 (c)(1), (2), (4) Date: Oct. 24, 2012 Sodium 2-(5-bromo-4-(4-cyclopropyl-naphthalen-1-yl)-4H Related U.S. Application Data 1,2,4-triazol-3-ylthio)acetate is described. In addition, phar (60) Provisional application No. 61/319,014, filed on Mar. maceutical compositions and uses Such compositions for the 30, 2010. treatment of a variety of diseases and conditions. Patent Application Publication Mar. 7, 2013 Sheet 1 of 10 US 2013/00598.68A1 FIGURE 1 S. C SOC 55000 40 S. s 3. s Patent Application Publication Mar. 7, 2013 Sheet 2 of 10 US 2013/00598.68A1 ~~~::CC©>???>©><!--->?©><??--~~~~~·%~~}--~~~~~~~~*~~~~~~~~·;--~~~~~~~~~;~~~~~~~~~~}--~~~~~~~~*~~~~~~~~;·~~~~~ |×.> |||—||--~~~~ ¿*|¡ MSU No IL-1ra MSU50 IL-1ra MSU 100 IL-1ra MSU500 IL-1ra cells Only No IL-1 ra Patent Application Publication Mar. 7, 2013 Sheet 3 of 10 US 2013/00598.68A1 FIGURE 3 A: 50000 40000 R 30000 2 20000 10000 O -7 -6 -5 -4 -3 Lesinurad (log)M B: Lesinurad (log)M Patent Application Publication Mar. -

PHARMACEUTICAL APPENDIX to the TARIFF SCHEDULE 2 Table 1
Harmonized Tariff Schedule of the United States (2020) Revision 19 Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE Harmonized Tariff Schedule of the United States (2020) Revision 19 Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 2 Table 1. This table enumerates products described by International Non-proprietary Names INN which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service CAS registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known. -

Qualitative Chemical Measures to Indirectly Assess Quality of Natural/Uncured, Organic Bacon Compared to Traditionally Cured Bacon
Iowa State University Capstones, Theses and Graduate Theses and Dissertations Dissertations 2009 Qualitative chemical measures to indirectly assess quality of natural/uncured, organic bacon compared to traditionally cured bacon. Charlwit Kulchaiyawat Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/etd Part of the Animal Sciences Commons Recommended Citation Kulchaiyawat, Charlwit, "Qualitative chemical measures to indirectly assess quality of natural/uncured, organic bacon compared to traditionally cured bacon." (2009). Graduate Theses and Dissertations. 10999. https://lib.dr.iastate.edu/etd/10999 This Thesis is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Graduate Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. Qualitative chemical measures to indirectly assess quality of natural/uncured, organic bacon compared to traditionally cured bacon by Charlwit Kulchaiyawat A thesis submitted to the graduate faculty in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE Major: Meat Science Program of Study Committee: Joseph Sebranek, Major Professor James Dickson Mark Honeyman Lester Wilson Iowa State University Ames, Iowa 2009 Copyright © Charlwit Kulchaiyawat 2009. All rights reserved. ii TABLE OF CONTENTS LIST OF TABLES iii ABSTRACT -

Guaiacol As a Drug Candidate for Treating Adult Polyglucosan Body Disease
Guaiacol as a drug candidate for treating adult polyglucosan body disease Or Kakhlon, … , Wyatt W. Yue, H. Orhan Akman JCI Insight. 2018;3(17):e99694. https://doi.org/10.1172/jci.insight.99694. Research Article Metabolism Therapeutics Graphical abstract Find the latest version: https://jci.me/99694/pdf RESEARCH ARTICLE Guaiacol as a drug candidate for treating adult polyglucosan body disease Or Kakhlon,1 Igor Ferreira,2 Leonardo J. Solmesky,3 Netaly Khazanov,4 Alexander Lossos,1 Rafael Alvarez,5 Deniz Yetil,6 Sergey Pampou,7 Miguel Weil,3,8 Hanoch Senderowitz,4 Pablo Escriba,5 Wyatt W. Yue,2 and H. Orhan Akman9 1Department of Neurology, Hadassah-Hebrew University Medical Center, Jerusalem, Israel. 2Structural Genomics Consortium, Nuffield Department of Clinical Medicine, University of Oxford, Oxford, United Kingdom.3 Cell Screening Facility for Personalized Medicine, Department of Cell Research and Immunology, The George S. Wise Faculty of Life Sciences, Tel Aviv University, Tel Aviv, Israel. 4Department of Chemistry, Bar Ilan University, Ramat Gan, Israel. 5Laboratory of Molecular Cell Biomedicine, Department of Biology, University of the Balearic Islands, Palma de Mallorca, Spain. 6Connecticut College, Newington, Connecticut USA. 7Columbia University Department of Systems Biology Irving Cancer Research Center, New York, New York, USA. 8Laboratory for Neurodegenerative Diseases and Personalized Medicine, Department of Cell Research and Immunology, The George S. Wise Faculty for Life Sciences, Sagol School of Neurosciences, Tel Aviv University, Ramat Aviv, Tel Aviv, Israel. 9Columbia University Medical Center Department of Neurology, Houston Merritt Neuromuscular diseases research center, New York, New York, USA. Adult polyglucosan body disease (APBD) is a late-onset disease caused by intracellular accumulation of polyglucosan bodies, formed due to glycogen-branching enzyme (GBE) deficiency. -

Metabolic Epoxidation Is a Critical Step for the Development of Benzbromarone-Induced Hepatotoxicity
DMD Fast Forward. Published on October 11, 2017 as DOI: 10.1124/dmd.117.077818 This article has not been copyedited and formatted. The final version may differ from this version. DMD # Title Page Metabolic epoxidation is a critical step for the development of benzbromarone-induced hepatotoxicity Hui Wang, Ying Peng, Tingjian Zhang, Qunsheng Lan, Huimin Zhao, Wenbao Wang, Yufei Zhao, Xu Wang, Jianxin Pang, Shaojie Wang, Jiang Zheng Wuya College of Innovation (H. W., Y. P., H. Z., Y. Z., X. W., J. Z.), Shenyang Pharmaceutical University, Shenyang, Liaoning, 11016, P. R. China; School of Pharmacy (T. Z.), China Medical University, Shenyang, Liaoning, 110122, P. R. Downloaded from China; Guangdong Provincial Key Laboratory of Drug Screening (Q. L., J. P.), School of Pharmaceutical Sciences, Southern Medical University, Guangzhou, Guangdong, 510515, P. R. China; Key Laboratory of Structure-Based Drug Design & Discovery (Ministry of Education) (W. W., S. W.), School of Pharmaceutical dmd.aspetjournals.org Engineering, Shenyang Pharmaceutical University, Shenyang, 110016, P. R. China; State Key Laboratory of Functions and Applications of Medicinal Plants (J. Z.), Key Laboratory of Pharmaceutics of Guizhou Province (J. Z.), Guizhou Medical University, Guiyang, Guizhou, 550004, P. R. China. at ASPET Journals on September 24, 2021 1 DMD Fast Forward. Published on October 11, 2017 as DOI: 10.1124/dmd.117.077818 This article has not been copyedited and formatted. The final version may differ from this version. DMD # Running Title Page Running title: Epoxidation and hepatotoxicity of benzbromarone Corresponding Authors: Jianxin Pang School of Pharmaceutical Sciences, Southern Medical University, 1838 North Guangzhou Ave, Guangzhou, Guangdong, 510515, China. -

Nitric Oxide.Vp
CHAPTER 5 Maple Leaf Foods, Toronto, Canada Brown Foundation Institute of Molecular Medicine, The University of Texas Health Science Center, Houston, TX 77030 ESPITE the many published reports on the beneficial properties of Dnitrite and nitrate in physiology, nitrite and nitrate in cured and pro- cessed meats continues to be perceived as harmful. The previous chapter revealed that certain foods, particularly green leafy vegetables are natu- rally enriched in nitrite and nitrate from growing in soil. However, en- riching meats with nitrite or nitrate during curing is perceived as harmful and advocated by some groups to be eliminated completely. The use of pure sodium nitrate in curing is now only a minor practice in the United States. The ingoing levels of sodium nitrite have been tightly controlled by the Food Safety and Inspection Service (FSIS) of the U.S. Depart- ment of Agriculture. To satisfy consumer demands for no added nitrite processed meats, efforts have recently been taken to creatively adjust the meat curing process by employing “nitrite free” organic vegetable pow- ders instead of directly adding sodium nitrite salts. Although the end re- sult is production of nitrite from the nitrate contained in the vegetable powders, a more “natural” or “organic” approach seems to appeal to con- sumers. This is primarily due to the public perception of nitrite and ni- trate. Reports about methemoglobinemia in infants (blue baby syndrome) caused by drinks or food prepared with nitrate-rich (and bac- terially contaminated) well water and vegetables, intentional and occu- pational intoxications in adults, increasing nitrate levels in soil and lakes as a result of fertilizer overuse, and the formation of potentially carcino- genic N-nitrosamines all contribute to the negative image that nitrite and nitrate have held in recent years. -

Letters to the Editor Benzbromarone Withdrawn from Table I
Letters to the Editor Benzbromarone withdrawn from Table I. A PubMed search (excluding Japanese papers) for fatal outcome using 4 treatment options: the European market: Another allopurinol, probenecid, benzbromarone, and sulfinpyrazone. case of "absence of evidence is No. of No. of Year of evidence of absence"? Fatal/death papers cases publication Allopurinola 14 14 1970-2001 Sirs, Probenecidb 1 1 1957 Gout, already described by Hippocrates, Benzbromaronec 2 3 1995-2000 has been one of the most curable disorders Sulfinpyrazoned 0 0 1976-2004 of modern rheumatology for years due to European registration status: aFully registered for gout. potent urate lowering therapeutic options. bRegistration previously held by MSD, withdrawn due to lack of profitability in the past, but often applicable on spe- While in only a minority of our rheumato- cial request. logically pre-selected patients xanthine oxi- cUp to 2003, registration in all of Europe, England excluded. From January 2004 registered only in Spain, and in The Netherlands for strict indications only, i.e. for allopurinol intolerant/allergic gouty patients. dRegistration held by dase inhibition by allopurinol lowered Novartis (Anturane®) only in England and Ireland, withdrawn from the market in Spain, not registered in other Euro- serum uric acid (SUA) levels sufficiently to pean countries and therefore generally not applicable. prevent gouty attacks, in non-preselected gouty patients 300 mg allopurinol normal- ized SUAin 85% of patients (1, 2). In most countries neither the uricosuric probenecid J.R.B.J. BROUWERS, Prof. Pharmacotherapy, cases gout is caused by inadequate, low uric nor sulfinpyrazone have been registered for PhD, Clinical pharmacologist acid excretion, explaining why uricosuric the treatment of gout. -

Report on the Deliberation Results May 8, 2013 Evaluation And
Report on the Deliberation Results May 8, 2013 Evaluation and Licensing Division, Pharmaceutical and Food Safety Bureau Ministry of Health, Labour and Welfare [Brand name] (a) Topiloric Tablets 20 mg, 40 mg, and 60 mg (b) Uriadec Tablets 20 mg, 40 mg, and 60 mg [Non-proprietary name] Topiroxostat (JAN*) [Applicant] (a) Fujiyakuhin Co., Ltd. (b) Sanwa Kagaku Kenkyusho Co., Ltd. [Date of application] June 26, 2012 [Results of deliberation] In the meeting held on April 26, 2013, the First Committee on New Drugs concluded that the product may be approved and that this result should be presented to the Pharmaceutical Affairs Department of the Pharmaceutical Affairs and Food Sanitation Council. The product is not classified as a biological product or a specified biological product, the re-examination period is 8 years, and neither the drug substance nor the drug product is classified as a poisonous drug or a powerful drug. *Japanese Accepted Name (modified INN) This English version of the Japanese review report is intended to be a reference material to provide convenience for users. In the event of inconsistency between the Japanese original and this English translation, the former shall prevail. The PMDA will not be responsible for any consequence resulting from the use of this English version. Review Report April 15, 2013 Pharmaceuticals and Medical Devices Agency The results of a regulatory review conducted by the Pharmaceuticals and Medical Devices Agency on the following pharmaceutical product submitted for registration are as follows. [Brand name] (a) Topiloric Tablets 20 mg, 40 mg, and 60 mg (b) Uriadec Tablets 20 mg, 40 mg, and 60 mg [Non-proprietary name] Topiroxostat [Applicant] (a) Fujiyakuhin Co., Ltd. -

The Use of Sodium Cyanide in Wildlife Damage Management
Human Health and Ecological Risk Assessment for the Use of Wildlife Damage Management Methods by USDA-APHIS-Wildlife Services Chapter VII THE USE OF SODIUM CYANIDE IN WILDLIFE DAMAGE MANAGEMENT May 2017 Peer Reviewed Final October 2019 THE USE OF SODIUM CYANIDE IN WILDLIFE DAMAGE MANAGEMENT EXECUTIVE SUMMARY USDA-APHIS Wildlife Services (WS) uses sodium cyanide (NaCN) to manage coyotes, red foxes, gray foxes, arctic foxes, and wild dogs that prey upon livestock, poultry, and federally designated threatened and endangered species or animals that are vectors of disease. This human health and ecological risk assessment is an evaluation of the risks to human health, nontarget animals, and the environment from NaCN use by WS. WS uses the M-44, the name for the ejector device that delivers a single dose NaCN from a capsule, to target canids. The M-44 is spring-activated and is actuated when an animal pulls up on the capsule holder; a plunger propelled by the spring breaks through a capsule with dry NaCN to deliver the contents into the mouth of an animal. The WS applicator baits the M-44 capsule holder sides to attract target canids. Sodium cyanide reacts rapidly with moisture in the mouth or mucus membranes of the nose and eyes to form hydrogen cyanide (HCN), a toxicant. One NaCN capsule contains enough cyanide to be lethal to animals through oral contact, inhalation contact, and moist dermal pathway contact. WS annually averaged the known take of 13,959 target canids and 362 nontarget species with NaCN between FY11 and FY15, recording 1,548,000 Method Nights with M-44s in 17 States.