Some Properties of a Virus from Galinsoga Parviflora*
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Metagenomic Approach to Understand Stand Failure in Bromus Tectorum
Brigham Young University BYU ScholarsArchive Theses and Dissertations 2019-06-01 A Metagenomic Approach to Understand Stand Failure in Bromus tectorum Nathan Joseph Ricks Brigham Young University Follow this and additional works at: https://scholarsarchive.byu.edu/etd BYU ScholarsArchive Citation Ricks, Nathan Joseph, "A Metagenomic Approach to Understand Stand Failure in Bromus tectorum" (2019). Theses and Dissertations. 8549. https://scholarsarchive.byu.edu/etd/8549 This Thesis is brought to you for free and open access by BYU ScholarsArchive. It has been accepted for inclusion in Theses and Dissertations by an authorized administrator of BYU ScholarsArchive. For more information, please contact [email protected], [email protected]. A Metagenomic Approach to Understand Stand Failure in Bromus tectorum Nathan Joseph Ricks A thesis submitted to the faculty of Brigham Young University in partial fulfillment of the requirements for the degree of Master of Science Craig Coleman, Chair John Chaston Susan Meyer Department of Plant and Wildlife Sciences Brigham Young University Copyright © 2019 Nathan Joseph Ricks All Rights Reserved ABSTACT A Metagenomic Approach to Understand Stand Failure in Bromus tectorum Nathan Joseph Ricks Department of Plant and Wildlife Sciences, BYU Master of Science Bromus tectorum (cheatgrass) is an invasive annual grass that has colonized large portions of the Intermountain west. Cheatgrass stand failures have been observed throughout the invaded region, the cause of which may be related to the presence of several species of pathogenic fungi in the soil or surface litter. In this study, metagenomics was used to better understand and compare the fungal communities between sites that have and have not experienced stand failure. -

View a Copy of This Licence, Visit
Hilton et al. Microbiome (2021) 9:19 https://doi.org/10.1186/s40168-020-00972-0 RESEARCH Open Access Identification of microbial signatures linked to oilseed rape yield decline at the landscape scale Sally Hilton1* , Emma Picot1, Susanne Schreiter2, David Bass3,4, Keith Norman5, Anna E. Oliver6, Jonathan D. Moore7, Tim H. Mauchline2, Peter R. Mills8, Graham R. Teakle1, Ian M. Clark2, Penny R. Hirsch2, Christopher J. van der Gast9 and Gary D. Bending1* Abstract Background: The plant microbiome plays a vital role in determining host health and productivity. However, we lack real-world comparative understanding of the factors which shape assembly of its diverse biota, and crucially relationships between microbiota composition and plant health. Here we investigated landscape scale rhizosphere microbial assembly processes in oilseed rape (OSR), the UK’s third most cultivated crop by area and the world's third largest source of vegetable oil, which suffers from yield decline associated with the frequency it is grown in rotations. By including 37 conventional farmers’ fields with varying OSR rotation frequencies, we present an innovative approach to identify microbial signatures characteristic of microbiomes which are beneficial and harmful to the host. Results: We show that OSR yield decline is linked to rotation frequency in real-world agricultural systems. We demonstrate fundamental differences in the environmental and agronomic drivers of protist, bacterial and fungal communities between root, rhizosphere soil and bulk soil compartments. We further discovered that the assembly of fungi, but neither bacteria nor protists, was influenced by OSR rotation frequency. However, there were individual abundant bacterial OTUs that correlated with either yield or rotation frequency. -

Plant Viruses Infecting Solanaceae Family Members In
Plant Viruses Infecting Solanaceae Family Members in the Cultivated and Wild Environments: A Review Richard Hančinský, Daniel Mihálik, Michaela Mrkvová, Thierry Candresse, Miroslav Glasa To cite this version: Richard Hančinský, Daniel Mihálik, Michaela Mrkvová, Thierry Candresse, Miroslav Glasa. Plant Viruses Infecting Solanaceae Family Members in the Cultivated and Wild Environments: A Review. Plants, MDPI, 2020, 9 (5), pp.667. 10.3390/plants9050667. hal-02866489 HAL Id: hal-02866489 https://hal.inrae.fr/hal-02866489 Submitted on 12 Jun 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Distributed under a Creative Commons Attribution| 4.0 International License plants Review Plant Viruses Infecting Solanaceae Family Members in the Cultivated and Wild Environments: A Review Richard Hanˇcinský 1, Daniel Mihálik 1,2,3, Michaela Mrkvová 1, Thierry Candresse 4 and Miroslav Glasa 1,5,* 1 Faculty of Natural Sciences, University of Ss. Cyril and Methodius, Nám. J. Herdu 2, 91701 Trnava, Slovakia; [email protected] (R.H.); [email protected] (D.M.); -

Clade (Kingdom Fungi, Phylum Chytridiomycota)
TAXONOMIC STATUS OF GENERA IN THE “NOWAKOWSKIELLA” CLADE (KINGDOM FUNGI, PHYLUM CHYTRIDIOMYCOTA): PHYLOGENETIC ANALYSIS OF MOLECULAR CHARACTERS WITH A REVIEW OF DESCRIBED SPECIES by SHARON ELIZABETH MOZLEY (Under the Direction of David Porter) ABSTRACT Chytrid fungi represent the earliest group of fungi to have emerged within the Kingdom Fungi. Unfortunately despite the importance of chytrids to understanding fungal evolution, the systematics of the group is in disarray and in desperate need of revision. Funding by the NSF PEET program has provided an opportunity to revise the systematics of chytrid fungi with an initial focus on four specific clades in the order Chytridiales. The “Nowakowskiella” clade was chosen as a test group for comparing molecular methods of phylogenetic reconstruction with the more traditional morphological and developmental character system used for classification in determining generic limits for chytrid genera. Portions of the 18S and 28S nrDNA genes were sequenced for isolates identified to genus level based on morphology to seven genera in the “Nowakowskiella” clade: Allochytridium, Catenochytridium, Cladochytrium, Endochytrium, Nephrochytrium, Nowakowskiella, and Septochytrium. Bayesian, parsimony, and maximum likelihood methods of phylogenetic inference were used to produce trees based on one (18S or 28S alone) and two-gene datasets in order to see if there would be a difference depending on which optimality criterion was used and the number of genes included. In addition to the molecular analysis, taxonomic summaries of all seven genera covering all validly published species with a listing of synonyms and questionable species is provided to give a better idea of what has been described and the morphological and developmental characters used to circumscribe each genus. -

Olive Mild Mosaic Virus Transmission by Olpidium Virulentus
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Repositório Científico da Universidade de Évora Eur J Plant Pathol DOI 10.1007/s10658-015-0593-z Olive mild mosaic virus transmission by Olpidium virulentus Carla M. R. Varanda & Susana Santos & Maria Ivone E. Clara & Maria do Rosário Félix Accepted: 7 January 2015 # Koninklijke Nederlandse Planteziektenkundige Vereniging 2015 Abstract The ability of Olpidium virulentus to vector The alphanecroviruses Olive mild mosaic virus Olive latent virus 1 (OLV-1), Olive mild mosaic virus (OMMV) and Olive latent virus 1 (OLV-1) and (OMMV) and Tobacco necrosis virus D (TNV-D) was the betanecrovirus Tobacco necrosis virus D evaluated. Transmission assays involved zoospore ac- (TNV-D) are very common in Portuguese olive quisition of each virus, inoculation onto cabbage plant orchards reaching infection levels of 31 % roots followed by viral detection. Assays revealed that (Varanda et al. 2006) and frequently appearing these viruses are transmitted in the absence of the fun- in mixed infections (Varanda et al. 2010). These gus, but the transmission rates of OMMV are much viruses are very similar and their differentiation higher when OMMV is incubated with O. virulentus is only possible through PCR - based assays zoospores prior to inoculation, while the transmission using specific primers (Varanda et al. 2010)or rates of each OLV-1 and TNV-D do not change when genome sequencing. they are incubated with the fungus. Our data shows that Prior to the discrimination into different species, O. virulentus is an efficient vector of OMMV, greatly TNV was found to be vectored by O. -

Toward a Fully Resolved Fungal Tree of Life
Annual Review of Microbiology Toward a Fully Resolved Fungal Tree of Life Timothy Y. James,1 Jason E. Stajich,2 Chris Todd Hittinger,3 and Antonis Rokas4 1Department of Ecology and Evolutionary Biology, University of Michigan, Ann Arbor, Michigan 48109, USA; email: [email protected] 2Department of Microbiology and Plant Pathology, Institute for Integrative Genome Biology, University of California, Riverside, California 92521, USA; email: [email protected] 3Laboratory of Genetics, DOE Great Lakes Bioenergy Research Center, Wisconsin Energy Institute, Center for Genomic Science and Innovation, J.F. Crow Institute for the Study of Evolution, University of Wisconsin–Madison, Madison, Wisconsin 53726, USA; email: [email protected] 4Department of Biological Sciences, Vanderbilt University, Nashville, Tennessee 37235, USA; email: [email protected] Annu. Rev. Microbiol. 2020. 74:291–313 Keywords First published as a Review in Advance on deep phylogeny, phylogenomic inference, uncultured majority, July 13, 2020 classification, systematics The Annual Review of Microbiology is online at micro.annualreviews.org Abstract https://doi.org/10.1146/annurev-micro-022020- Access provided by Vanderbilt University on 06/28/21. For personal use only. In this review, we discuss the current status and future challenges for fully 051835 Annu. Rev. Microbiol. 2020.74:291-313. Downloaded from www.annualreviews.org elucidating the fungal tree of life. In the last 15 years, advances in genomic Copyright © 2020 by Annual Reviews. technologies have revolutionized fungal systematics, ushering the field into All rights reserved the phylogenomic era. This has made the unthinkable possible, namely ac- cess to the entire genetic record of all known extant taxa. -

High-Level Classification of the Fungi and a Tool for Evolutionary Ecological Analyses
Fungal Diversity (2018) 90:135–159 https://doi.org/10.1007/s13225-018-0401-0 (0123456789().,-volV)(0123456789().,-volV) High-level classification of the Fungi and a tool for evolutionary ecological analyses 1,2,3 4 1,2 3,5 Leho Tedersoo • Santiago Sa´nchez-Ramı´rez • Urmas Ko˜ ljalg • Mohammad Bahram • 6 6,7 8 5 1 Markus Do¨ ring • Dmitry Schigel • Tom May • Martin Ryberg • Kessy Abarenkov Received: 22 February 2018 / Accepted: 1 May 2018 / Published online: 16 May 2018 Ó The Author(s) 2018 Abstract High-throughput sequencing studies generate vast amounts of taxonomic data. Evolutionary ecological hypotheses of the recovered taxa and Species Hypotheses are difficult to test due to problems with alignments and the lack of a phylogenetic backbone. We propose an updated phylum- and class-level fungal classification accounting for monophyly and divergence time so that the main taxonomic ranks are more informative. Based on phylogenies and divergence time estimates, we adopt phylum rank to Aphelidiomycota, Basidiobolomycota, Calcarisporiellomycota, Glomeromycota, Entomoph- thoromycota, Entorrhizomycota, Kickxellomycota, Monoblepharomycota, Mortierellomycota and Olpidiomycota. We accept nine subkingdoms to accommodate these 18 phyla. We consider the kingdom Nucleariae (phyla Nuclearida and Fonticulida) as a sister group to the Fungi. We also introduce a perl script and a newick-formatted classification backbone for assigning Species Hypotheses into a hierarchical taxonomic framework, using this or any other classification system. We provide an example -

Plant Viruses Infecting Solanaceae Family Members in the Cultivated and Wild Environments: a Review
plants Review Plant Viruses Infecting Solanaceae Family Members in the Cultivated and Wild Environments: A Review Richard Hanˇcinský 1, Daniel Mihálik 1,2,3, Michaela Mrkvová 1, Thierry Candresse 4 and Miroslav Glasa 1,5,* 1 Faculty of Natural Sciences, University of Ss. Cyril and Methodius, Nám. J. Herdu 2, 91701 Trnava, Slovakia; [email protected] (R.H.); [email protected] (D.M.); [email protected] (M.M.) 2 Institute of High Mountain Biology, University of Žilina, Univerzitná 8215/1, 01026 Žilina, Slovakia 3 National Agricultural and Food Centre, Research Institute of Plant Production, Bratislavská cesta 122, 92168 Piešt’any, Slovakia 4 INRAE, University Bordeaux, UMR BFP, 33140 Villenave d’Ornon, France; [email protected] 5 Biomedical Research Center of the Slovak Academy of Sciences, Institute of Virology, Dúbravská cesta 9, 84505 Bratislava, Slovakia * Correspondence: [email protected]; Tel.: +421-2-5930-2447 Received: 16 April 2020; Accepted: 22 May 2020; Published: 25 May 2020 Abstract: Plant viruses infecting crop species are causing long-lasting economic losses and are endangering food security worldwide. Ongoing events, such as climate change, changes in agricultural practices, globalization of markets or changes in plant virus vector populations, are affecting plant virus life cycles. Because farmer’s fields are part of the larger environment, the role of wild plant species in plant virus life cycles can provide information about underlying processes during virus transmission and spread. This review focuses on the Solanaceae family, which contains thousands of species growing all around the world, including crop species, wild flora and model plants for genetic research. -
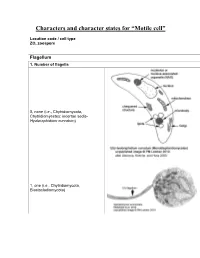
Characters and Character States for “Motile Cell”
Characters and character states for “Motile cell” Location code / cell type ZO, zoospore Flagellum 1. Number of flagella 0, none (i.e., Chytridiomycota, Chytridiomycetes: incertae sedis- Hyaloraphidium curvatum) 1, one (i.e., Chytridiomycota, Blastocladiomycota) 2, multiple (i.e., Neocallimastigomycota) 2. Electron-opaque plug in axoneme core and between axoneme and flagellar membrane 0, absent 1, present (i.e., Chytridiomycota: Chytridiomycetes: Chytridiales, Lobulomycetales, Cladochytriales, incertae sedis: Synchytrium endobioticum, Polychytrium clade; Monoblepharidomycetes) 3. Flagellum coating 0, absent (all taxa except Polyphagus euglenae) 1, present (Polyphagus euglenae) Kinetosome (the term “basal body” is synonymous; Andersen et al. 1991) 4. Electron-opaque core in kinetosome 0, absent (all taxa except Kappamyces) 1, present (Chytridiomycota: Chytridiomycetes: Rhizophydiales- Kappamyces) 5. Scalloped ring within kinetosome, extensions of the A, B, or C microtubule 0, absent 1, present (Lacustromyces hiemalis) Kinetosome-associated structures 6. Kinetosome support 0, absent (Thalassochytrium gracilaripsidis) 1, kinetosome props 2, broken kinetosome props (Olpidium radicale) 3, skirt-like structure surrounding kinetosome (Neocallimastigomycota) 7. Kinetosome-associated plates 0, absent 1, present (Chytridiomycota: Chytridiomycetes: Chytridiales- Group I- type zoospore [Barr 1980]) 8. Kinetosome-associated saddle 0, absent 1, present (Chytridiomycota: Chytridiomycetes: Chytridiales- Group II-type zoospore [Barr 1980]- Chytridium -

Discussion of Phylogenetic Relationships
Discussion of Phylogenetic Relationships The kingdom Fungi is a diverse clade of heterotrophic organisms that shares some characters with animals such as chitinous structures, storage of glycogen, and mitochondrial codon UGA encoding tryptophan. Both animals and fungi have spores or gametes with a single smooth, posteriorly inserted flagellum, but only species of the basal chytrid phyla have retained this primitive character (Barr, 1992; Cavalier-Smith, 1987, 1995). Fungi, animals, and other heterotrophic protist-like organisms such as choanoflagellates and Mesomycetozoea are now considered part of the larger group termed opisthokonts (Cavalier-Smith, 1987) in reference to the posterior flagellum. The branch uniting the fungi and animals is well-supported based on a number of molecular phylogenetic datasets, including the nuclear small subunit ribosomal RNA gene (Wainwright et al., 1993; Bruns et al. 1993), unique and shared sequence insertions in proteins such as elongation factor 1α (Baldauf and Palmer, 1993), entire mitochondrial genomes (Lang et al., 2002), and concatenated protein-coding genes (Steenkamp et al., 2006). Prior classification systems of Fungi based primarily on morphology are in need of updating to more accurately reflect phylogenetic relationships as determined by molecular systematics. Molecular characters have been essential for phylogenetic analysis in cases when morphological characters are convergent, reduced, or missing among the taxa considered. This is especially true of species that never reproduce sexually, because characters of sexual reproduction traditionally have been the basis for classification of Fungi. Use of molecular characters allows asexual fungi to be placed among their closest relatives. Previous classifications placed early-diverging fungal groups (non-Ascomycota or Basidiomycota) into two phyla: Chytridiomycota and Zygomycota. -

Biological Control by Nematophagous Fungi for Plant-Parasitic Nematodes in Soils
ISSN 0367-6315 Korean J. Soil Sci. Fert. 45(1), 74-78 (2012) Article Biological Control by Nematophagous Fungi for Plant-parasitic Nematodes in Soils Jun-hyeong Park, Sun-jung Kim, Jin-ho Choi, Min-ho Yoon, Doug-young Chung, and Hye-Jin Kim* Departmnet of Bio-environmental Chemistry, College of Agriculture and Life Science, Chungnam National University Daejeon Korea 305-764 Envioronmental concerns by use of chemical pesticides have increased the need for alternative method in the control of plant-parasitic nematodes. Biological control is considered eco-friendly and a promising alternative in pest and disease management. A wide range of organisms are known to be effective in control of plant-parasitic nematodes. Fungal biological control is a hopeful research area and there is constant attention in the use of fungi for the control of nematodes. In this review, plant-parasitic nematodes with reference to soils and biological control and nematophagous fungi are dicussed. Key words: Biological control, Nematophagous fungi, Plant-parasitic nematodes, Soil Introduction but most interest has been focused on nematophagous fungi, especially those that restrain their hosts with specialized traps (Kerry, 1990). Since the discovery Nematodes, water-filled pore spaces in the soil where of nematode-trapping fungi (Linford and Yap, 1939), organic matter, plant roots, and resources are most a lot of study about nematophagous fungi had been plentiful, are microscopic, worm-like organisms that conducted and still has been carrying out for nematocidal inhabit water films. nematode, most abundant in the agents. In this paper, recent advances in the field of upper soil layers, generally ranges from one to ten -2 biological control of plant-parasitic nematodes with million individuals m (Peterson and Luxton, 1982; nematophagous fungi will be evaluated. -

PCR Detection of Olpidium Brassicae and Differentiation of Lettuce-Infecting Isolates from Brassica-Infecting Isolates
University of Warwick institutional repository This paper is made available online in accordance with publisher policies. Please scroll down to view the document itself. Please refer to the repository record for this item and our policy information available from the repository home page for further information. To see the final version of this paper please visit the publisher’s website. Access to the published version may require a subscription. Author(s): Lucy M. HARTWRIGHT, Paul J. HUNTER, John A. WALSH Article Title: A comparison of Olpidium isolates from a range of host plants using internal transcribed spacer sequence analysis and host range studies Year of publication: 2009 Link to published version : http://dx.doi.org/10.1016/j.mycres.2009.09.008 Publisher statement: None A comparison of Olpidium isolates from a range of host plants using internal transcribed spacer sequence analysis and host range studies. Lucy M. HARTWRIGHT, Paul J. HUNTER and John A. WALSH* Warwick HRI, University of Warwick, Wellesbourne, Warwick, CV35 9EF, UK. *Corresponding author. Fax: +44 (0)2476 574500 Telephone: +44 (0)2476 575028 E-mail address: [email protected] Running title: Olpidium brassicae host types Keywords: Fungus Chytridiomycete Virus vector PCR ABSTRACT Olpidium brassicae is a ubiquitous obligate root-infecting fungal pathogen. It is an important vector of a wide range of plant viruses. Olpidium isolates that infected brassica plants did not infect lettuce plants and vice-versa. Host range tests, PCR amplification and sequencing of the internal transcribed spacer (ITS) and 5.8S regions of 25 Olpidium isolates from brassica, carrot, cucumber and lettuce originating from four continents revealed differences between isolates.