Riparian Ecosystems and Their Management: Reconciling
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

CENTRAL ARIZONA SALINITY STUDY --- PHASE I Technical Appendix C HYDROLOGIC REPORT on the PHOENIX
CENTRAL ARIZONA SALINITY STUDY --- PHASE I Technical Appendix C HYDROLOGIC REPORT ON THE PHOENIX AMA Prepared for: United States Department of Interior Bureau of Reclamation Prepared by: Brown and Caldwell 201 East Washington Street, Suite 500 Phoenix, Arizona 85004 Brown and Caldwell Project No. 23481.001 C-1 TABLE OF CONTENTS PAGE TABLE OF CONTENTS ................................................................................................................ 2 LIST OF TABLES .......................................................................................................................... 3 LIST OF FIGURES ........................................................................................................................ 3 1.0 INTRODUCTION .............................................................................................................. 4 2.0 PHYSICAL SETTING ....................................................................................................... 5 3.0 GENERALIZED GEOLOGY ............................................................................................ 6 3.1 BEDROCK GEOLOGY ......................................................................................... 6 3.2 BASIN GEOLOGY ................................................................................................ 6 4.0 HYDROGEOLOGIC CONDITIONS ................................................................................ 9 4.1 GROUNDWATER OCCURRENCE .................................................................... -

Mineral Resources of the Harquahala Mountains Wilderness Study Area, La Paz and Maricopa Counties, Arizona
2.SOB nH in ntoiOGIGM. JAN 3 1 1989 Mineral Resources of the Harquahala Mountains Wilderness Study Area, La Paz and Maricopa Counties, Arizona U.S. GEOLOGICAL SURVEY BULLETIN 1701-C Chapter C Mineral Resources of the Harquahala Mountains Wilderness Study Area, La Paz and Maricopa Counties, Arizona By ED DE WITT, S.M. RICHARD, J.R. HASSEMER, and W.F. HANNA U.S. Geological Survey J.R. THOMPSON U.S. Bureau of Mines U.S. GEOLOGICAL SURVEY BULLETIN 1701 MINERAL RESOURCES OF WILDERNESS STUDY AREAS- WEST-CENTRAL ARIZONA AND PART OF SAN BERNARDINO COUNTY, CALIFORNIA U. S. GEOLOGICAL SURVEY Dallas L Peck, Director UNITED STATES GOVERNMENT PRINTING OFFICE: 1988 For sale by the Books and Open-File Reports Section U.S. Geological Survey Federal Center Box 25425 Denver, CO 80225 Library of Congress Cataloging-in-Publlcatlon Data Mineral resources of the Harquahala Mountains wilderness study area, La Paz and Maricopa counties, Arizona. (Mineral resources of wilderness study areas west-central Arizona and part of San Bernardino County, California ; ch. C) (U.S. Geological Survey bulletin ; 1701-C) Bibliography: p. Supt. of Docs, no.: I 19.3:1701-C 1. Mines and mineral resources Arizona Harquahala Mountains Wilderness. 2. Harquahala Mountains (Ariz.) I. DeWitt, Ed. II. Series. III. Series: U.S. Geological Survey bulletin ; 1701. QE75.B9 no. 1701-C 557.3 s [553'.09791'72] 88-600012 [TN24.A6] STUDIES RELATED TO WILDERNESS Bureau of Land Management Wilderness Study Areas The Federal Land Policy and Management Act (Public Law 94-579, October 21, 1976) requires the U.S. Geological Survey and the U.S. -

The Maricopa County Wildlife Connectivity Assessment: Report on Stakeholder Input January 2012
The Maricopa County Wildlife Connectivity Assessment: Report on Stakeholder Input January 2012 (Photographs: Arizona Game and Fish Department) Arizona Game and Fish Department In partnership with the Arizona Wildlife Linkages Workgroup TABLE OF CONTENTS LIST OF FIGURES ............................................................................................................................ i RECOMMENDED CITATION ........................................................................................................ ii ACKNOWLEDGMENTS ................................................................................................................. ii EXECUTIVE SUMMARY ................................................................................................................ iii DEFINITIONS ................................................................................................................................ iv BACKGROUND ................................................................................................................................ 1 THE MARICOPA COUNTY WILDLIFE CONNECTIVITY ASSESSMENT ................................... 8 HOW TO USE THIS REPORT AND ASSOCIATED GIS DATA ................................................... 10 METHODS ..................................................................................................................................... 12 MASTER LIST OF WILDLIFE LINKAGES AND HABITAT BLOCKSAND BARRIERS ................ 16 REFERENCE MAPS ....................................................................................................................... -

Geochronology, Geology, and Listric Normal Faulting of the Vulture Mountains, Maricopa County, Arizona
Arizona Geological Society Digest, Volume XII, 1980 89 Geochronology, Geology, and Listric Normal Faulting of the Vulture Mountains, Maricopa County, Arizona by WA. Rehrigi, M. Shafiqullah2, and P.E. Damon2 Abstract Geologic mapping and geochronologic studies in the Vulture Mountains near Wickenburg, Arizona, have led to the recognition of a large, northeast-trending batholith of 68.4-m.y. age that intrudes complex gneissic and granitic rocks of probably Precambrian age. Over- lying the denuded crystalline terrane is a sequence of late Oligocene to Miocene ( .'26 to 16 m.y.) volcanic rocks (vitrophyres, ash-flow tuffs, welded tuffs, breccias, agglomerates, and lava flows) that vary locally. Nearby source areas are suggested. A swarm of north- to north-northwest-trending porphyritic dikes intrudes the volcanics and crystalline basement. Overlying this volcanic sequence in angular unconformity is a thin section of basal conglom- erate and basalt lava flows dated at 13.5 m.y. B.P. The older, tuffaceous sequence is generally calc-alkalic but with a high proportion of rhyolites that are exceptionally rich in potassium and silica. These silicic units are peral- kaline or nearly so, and those with K20/Na2O >3 are ultrapotassic. Initial strontium ratios average 0.7081, whereas an initial ratio for the younger basalt sequence is significantly lower at 0.7054. The silicic volcanics have been severely tilted on multiple, low-angle listric normal faults. The youngest basalt flows are relatively flat lying and postdate this deformation. By geo- logic and radiometric criteria, the transition from tilted silicic volcanics to untilted basalts occurred between about 16 and 14 m.y. -

Detailed Geologic Map and Cross Sections of the Big Horn And
GEOLOGIC MAP AND CROSS SECTIONS OF THE BIG HORN AND BELMONT MOUNTAINS, WEST-CENTRAL ARIZONA James A. Stin1ac, Stephen M. Richard, Stephen J. Reynolds, Richard C. Capps, Curtis P. Kortel11eier, Michael J. Grubensky, George B. Allen, Floyd Gray, and Robert J. Miller Open-File Report 94-15 ARIZONA GEOLOGICAL SURVEY TUCSON, ARIZONA NOVEIVIBER, 1994 This report is preliminary and lias not been edited or reviewed for conformity with Arizona Geological Survey standards INTRODUCTION The principal geologic feature of the Big Hom and Belmont Mountains is a complexly faulted and tilted series of mostly Miocene volcanic rock that record a period of Middle Tertiary magmatism and extension. These volcanic rocks vary widely in composition, but basaltic and rhyolitic rocks are most abundant (Figure 1). Intrusive equivalents of these volcanics exposed in the Belmont Mountains are dominantly granitic. Despite the large volume of rhyolite erupted, small, coalescing flow and dome complexes were formed in preference to large- MIocene and Oligocene sandstone and conglomerate Hot Rock basalt Burnt Mountain volcanics Big Horn volcanics Intrusive rocks pre-Tertiary rocks I~ ,:- ; ~ _I Big Horn granodiorite (Cretaceous) ~ Igneous and metamorpchic rocks ~ (Proterozoic through Mesozoic) Figure 1. Generalized lithologic map of the Big Hom and Belmont Mountains volume ash-flow tuffs, and no collapse calderas were formed. These rocks lie in the upper plate ofthe regional Whipple-Buckskin-Bullard detachment fault [Rehrig and Reynolds, 1980], at its southeastern tip [Richard et aI, 1990a]. A regional boundary between major tilts domains in Tertiary strata follows an irregular course from northwest to southeast through the range [Rehrig et aI., 1980]. -

Arizona's Wildlife Linkages Assessment
ARIZONAARIZONA’’SS WILDLIFEWILDLIFE LINKAGESLINKAGES ASSESSMENTASSESSMENT Workgroup Prepared by: The Arizona Wildlife Linkages ARIZONA’S WILDLIFE LINKAGES ASSESSMENT 2006 ARIZONA’S WILDLIFE LINKAGES ASSESSMENT Arizona’s Wildlife Linkages Assessment Prepared by: The Arizona Wildlife Linkages Workgroup Siobhan E. Nordhaugen, Arizona Department of Transportation, Natural Resources Management Group Evelyn Erlandsen, Arizona Game and Fish Department, Habitat Branch Paul Beier, Northern Arizona University, School of Forestry Bruce D. Eilerts, Arizona Department of Transportation, Natural Resources Management Group Ray Schweinsburg, Arizona Game and Fish Department, Research Branch Terry Brennan, USDA Forest Service, Tonto National Forest Ted Cordery, Bureau of Land Management Norris Dodd, Arizona Game and Fish Department, Research Branch Melissa Maiefski, Arizona Department of Transportation, Environmental Planning Group Janice Przybyl, The Sky Island Alliance Steve Thomas, Federal Highway Administration Kim Vacariu, The Wildlands Project Stuart Wells, US Fish and Wildlife Service 2006 ARIZONA’S WILDLIFE LINKAGES ASSESSMENT First Printing Date: December, 2006 Copyright © 2006 The Arizona Wildlife Linkages Workgroup Reproduction of this publication for educational or other non-commercial purposes is authorized without prior written consent from the copyright holder provided the source is fully acknowledged. Reproduction of this publication for resale or other commercial purposes is prohibited without prior written consent of the copyright holder. Additional copies may be obtained by submitting a request to: The Arizona Wildlife Linkages Workgroup E-mail: [email protected] 2006 ARIZONA’S WILDLIFE LINKAGES ASSESSMENT The Arizona Wildlife Linkages Workgroup Mission Statement “To identify and promote wildlife habitat connectivity using a collaborative, science based effort to provide safe passage for people and wildlife” 2006 ARIZONA’S WILDLIFE LINKAGES ASSESSMENT Primary Contacts: Bruce D. -

Geologic Map of the Wickenburg, Southern Buckhorn, and Northwestern Hieroglyphic Mountains, Central Arizona
Geologic Map of the Wickenburg, southern Buckhorn, and northwestern Hieroglyphic Mountains, central Arizona _ by James A. Stimac, Joan E. Fryxell. Stephen J. Reynolds, Stephen M. Richard, Michael J. GrubenskY, and Elizabeth A. Scott Arizona Geological Survey Open-File Report 87-9 October, 1987 Arizona Geological Survey 416 W. Congress, Suite #100, Tucson, Arizona 85701 This report is preliminalY and has not been edited or reviewed for conformity with Arizona Geological Survey standards INTRODUCTION This report describes the geology of the Red Picacho quadrangle and parts of the Wickenburg, Garfias Mountain, and Wittmann quadrangles (Fig. 1). Geologic mapping was completed between January and April of 1987, and was jointly funded by the U.S. Geological Survey and the Arizona Bureau of Geology and Mineral Technology as part of the cost-sharing COGEOMAP program. Mapping was done on 1:24,000-scale topographic maps and on 1:24,000-scale color aerial photographs provided by Raymond A. Brady of the U.S. Bureau of Land Management. GEOLOGIC OVERVIEW The map area includes the Wickenburg Mountains and contiguous parts of the Buckhorn and Hieroglyphic Mountains (Fig. 1). Adjacent parts of the Vulture Mountains were mapped by Grubensky and oth_ers (1987) and adjacent parts of the Hieroglyphic Mountains were mapped by Capps and others (1986). The overall geologic history of the area is complex, but the regional stratigraphy developed in these reports carries well from range to range. The map area is composed of a metamorphic-plutonic basement unconformably overlain by Tertiary volcanic and sedimentary rocks. The oldest rocks, assigned to the Proterozoic (1.8-1.7 b.y.) Yavapai Supergroup, consist of amphibolite, schist, and gneiss, intruded by granite, leucogranite, and pegmatite. -

Tonopah Arlington Area Plan
AREA PLAN TO N O PAH /ARLIN GTO N 2020 Eye To Th e Fut u re Executive Summary OVERVIEW The Area Plan process is structured to emphasize public involvement and incorporate citizen comments, ideas, and direction into the plan. The Maricopa County Planning and Development Department began the Tonopah/Arlington Area Plan update in the winter of 1997. The update process involved many individuals and organizations from the Tonopah/ Arlington planning area. Central to the planning process a citizen steering committee was formed, two open houses were held in the summer and fall of 1998 and another in the fall of 1999, and focus group sessions addressed specific community issues. WHAT’S NEW IN THE PLAN? 1. Land use categories that follow the regional standard and are consistent with the Comprehensive Plan. 2. Additional land use categories for residential, resort, and industrial. 3. Discussion and analysis of current issues. 4. Redesigned and rewritten Area Plan with updated digital maps. 5. Tonopah and Arlington are discussed as individual areas. HOW TO USE THE PLAN The Tonopah/Arlington Area Plan provides a specific guide for decisions by the Planning and Zoning Commission and the Board of Supervisors concerning growth and development in the Tonopah/Arlington planning area. It is to be used by policy makers to guide their decisions and serve as a reference for private sector decision making. AREA PLAN ELEMENTS The Area Plan elements contain a series of goals, objectives and policies used to define development standards, guide public investment, and public and private decision making. LAND USE The land use designations in this Area Plan embody generalized land use, development or preservation concepts. -

Dismembered Porphyry Systems Near Wickenburg, Arizona: District-Scale Reconstruction with an Arc-Scale Context
©2016 Society of Economic Geologists, Inc. Economic Geology, v. 111, pp. 447–466 Dismembered Porphyry Systems near Wickenburg, Arizona: District-Scale Reconstruction with an Arc-Scale Context Phillip A. Nickerson†,* and Eric Seedorff Lowell Institute for Mineral Resources, Department of Geosciences, University of Arizona, 1040 East Fourth Street, Tucson, Arizona 85721-0077 Abstract This study combines results from reconnaissance-scale mapping of hydrothermal alteration, rock types, and structures to provide a district-scale cross section and associated palinspastic reconstruction of an area with two previously undescribed Laramide (~70 Ma) porphyry systems at Sheep Mountain and Copper Basin (Crown King). Extension at the district scale is placed in an arc-scale context using an original compilation of strike and dip measurements on Tertiary rocks to reconstruct the Laramide porphyry belt prior to extension. The study area contains five sequential, partially superimposed sets of normal faults that are nearly planar where exposed. Dips of all normal faults initiated at 60° to 70° and rotated during slip to angles as gentle as 20°. A palinspastic reconstruction reveals that two, spatially distinct hydrothermal systems overlie different cupolas of a Late Cretaceous pluton. Hydrothermal alteration is zoned from greisen to potassic to transitional greisen- potassic assemblages from deep to shallow structural levels. The reconstruction is used to identify two covered exploration targets. The prospects may be porphyry molybdenum systems of the quartz monzonitic-granitic porphyry Mo-Cu subclass, joining others in an arc that is best known for porphyry copper deposits. The Laramide porphyry belt prior to extension displays a variably well-defined axis, ~100 km wide, with gaps and clusters of deposits along its 700-km strike length. -
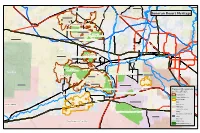
Sonoran Desert Heritage
Aguila «¬71 ¤£60 MAZATZAL Wilderness HELLS CANYON Wilderness Gladden Eagle Eye Mountain # Vulture Peak # Salome Peak # Morristown Sonoran Desert HerTionttoa Ng.F e Black Butte # «¬72 HARQUAHALA MTNS. Wilderness Vulture «¬74 Belmont - Harquahala NCA Hummingbird Springs Wilderness HUMMINGBIRD SPRINGS Wilderness Tonopah-Belmont Mine ¨¦§10 Belmont Mountains Wilderness BIG HORN MTNS. Wilderness NEW WATER MTNS. Wilderness «¬101 Hummingbird Springs Wilderness «¬303 Peoria Burnt Mountain 60 # ¤£ «¬51 Luke A.F.B Glendale ¨¦§17 87 Courthouse Rock «¬ # 143 Phoenix «¬ # Wintersburg Gila Bend NCA ¨¦§10 EAGLETAIL MTNS Wilderness # Saddle Mountain Centennial Wilderness Liberty £60 Buckeye ¤ Estrella Mountain Ranch Arlington «¬202 Clanton Hills # Yellow Medicine Hills Columbus Peak # # 101 Cortez Peak # «¬ # Dixie Peak Gila Bend Wilderness # Black Mountain Kofa N.W.R # Webb Mountain # Sonoran Desert National Monument Wilderness SIERRA ESTRELLA Wilderness Gila Bend NCA SIGNAL MTN. Wilderness Saddle Enterprise Ranch «¬587 Gila Bend NCA Rainbow Valley SMA # WOOLSEY PEAK Wilderness KOFA REFUGE Wilderness NORTH MARICOPA MTNS. Wilderness «¬85 Painted Rock Dam Oatman Mountain # Sonoran Desert N.M. Woolsey Peak Wilderness Addition Proposed Designatio«¬87 ns Hyder ENSCO Site and Land Tenure Oatman Flat Sonoran Desert National Monument Wilderness Agua Caliente Proposed NCA «¬387 Proposed Wilderness Gila Bend Proposed SMA «¬187 Paloma Ranch SOUTH MARICOPA MTNS. Wilderness BLM Sentinel Plain SMA Yuma Test Range ¨¦§8 Forest Service Sentinel «¬287 «¬84 Tribal Land Local or State Parks Sonoran Desert National Monument Wilderness Military Natl. Monument (BLM land) TABLE TOP Wilderness Other State Trust Barry Goldwater Air Force Rng. WToilhdolifneo L aInnddian Res. Existing Wilderness Area Copyright:© 2009 ESRI. -

White Tank Mountain Regional Park
White Tank Mountain Regional Park Master Plan Update 2014-2034 {This page intentionally left blank.} {This page intentionally left blank.} {This page intentionally left blank.} Acknowledgements This master plan update was a collaborative process that involved the guidance and expertise of many. The Maricopa County Parks and Recreation Department would like to thank the Planning Team who committed their time to monthly meetings and document review; likewise to the Stakeholder Advisory Group who took time out of their personal lives to provide their invaluable input. Planning Team Stakeholder Advisory Group R.J. Cardin, Director Jamil Coury Jennifer Waller, Operations Manager Linda Gilgosch Jennifer Johnston, West Side Superintendent John Pesock Raymond Schell, Park Supervisor Frank Salowitz Michele Kogl, Planning and Development Manager Allen Ockenfels, Trails Development Manager Fareed Abou-Haidar, GIS Technician Leigh Johnson, Park Planner This Master Plan update was also made possible by the contributions and guidance of the following: • Maricopa County Board of Supervisors o Denny Barney, District 1 o Steve Chucri, District 2 o Andy Kunasek, District 3 o Clint L. Hickman, District 4 o Mary Rose Wilcox, District 5 • Maricopa County Parks and Recreation Commission o Jack Stapley, District 2 o Anne Lynch, District 3 o Dr. Robert Branch, District 4 o Carlton Yoshioka , Member-at-Large o Rod Jarvis, Member-at-Large • Maricopa County Parks and Recreation Department staff • Maricopa County Sheriff’s Office, Mountain Patrol Division • Bill “Doc” Talboys, Interpretive Ranger, MCPRD (retired) • Shelly Rasmussen, Interpretive Ranger, MCPRD (retired) • Public meeting participants The Department would also like to thank its agency partners at the City of Surprise, City of Buckeye, Arizona Game and Fish Department, and the Bureau of Land Management - Phoenix District Office for their input and guidance. -

Compilation Geologic Map of the Daisy Mountain 7.5' Quadrangle, Maricopa County, Arizona
Compilation Geologic Map of the Daisy Mountain 7.5' Quadrangle, Maricopa County, Arizona by Robert S. Leighty Arizona Geological Survey Open-File Report 98-22 August, 1998 Arizona Geological Survey 416 W. Congress, Suite 100, Tucson, AZ 85701 Includes 30 page text and 1 :24,000 scale geologic map. This report was supported by the Arizona Radiation Regulatory Agency, with funds provided by the u.s. Environmental Protection Agency through the State Indoor Radon Grant Program, the U.S. Geological Survey via the STATEMAP program, and the Arizona Geological Survey. This report is preliminary and has not been edited or reviewed for conformity with Arizona Geological Survey standards INTRODUCTION The Daisy Mountain Quadrangle, located along the northernmost fringe of the Phoenix metropolitan area, straddles the physiographic boundary between the Basin and Range and Transition Zone (Figure 1). The quadrangle lies between 1-17 and Cave Creek and is bordered on the south by the northwestern end of Paradise Valley and rugged, high-relief terrain of the New River Mountains to the north. The Daisy Mountain Quadrangle includes the community of New River, which is undergoing rapid population growth and is becoming increasingly urbanized. Thus, the knowledge of the distribution and character of bedrock and surficial deposits is important to make informed decisions concerning management of the land and its resources. This project was funded by the Environmental Protection Agency through the State Indoor Radon Grant Program, and the Arizona Geological Survey. PREVIOUS STUDIES Over the last two decades various workers have conducted geologic mapping investigations in the Daisy Mountain Quadrangle.